Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
- CAS No.
- 9004-64-2
- EC No.
- 618-388-0
- Technologies
- Product Families
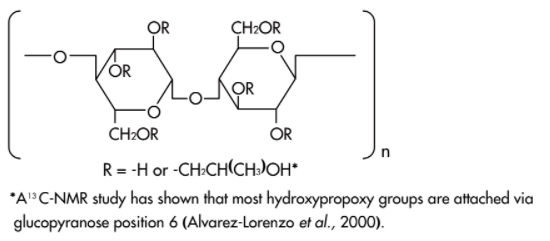
- Structure
Features & Benefits
- Labeling Claims
- Features
- Water insoluble, swells in water and works as dual functional ingredients, disintegrant and binder for tablets and pellets.
- Suitable grade can be selected depending on process and API characteristics.
- Non-ionic polymer which has less interaction with API and better stability.
Applications & Uses
Properties
- Physical Form
- Typical Properties
Value Units Test Method / Conditions Chloride min. 0.36 % USP/NF Heavy Metals min. 0.001 % USP/NF Hydroxypropoxy Content 11.0 % - Hydroxypropoxy Content 10.0 - 12.9 % USP/NF Loss on drying min. 5.0 % USP/NF Particle Morphology Micronized - - pH 5.0 - 7.5 - JP Residue on ignition Not more than 0.5 % USP/NF - Particle Size Distribution
Value Units Test Method / Conditions Mean Particle Size 20.0 μm - Particle Size (90% cumulated) 40 - 100 μm Shin-Etsu Particle Size (Average) 17 - 23 μm Shin-Etsu - Note
*In-house Laser diffraction method
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Grade
Technical Details & Test Data
- Anti-Capping Effect of L-HPC
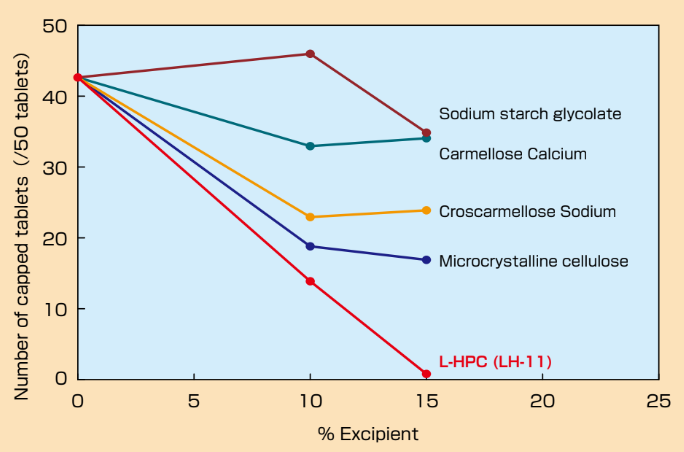
Ethenzamide tablets were prepared with various ratio of excipients and friability test was implemented in accordance with USP method.
- Pictures of Aspirin Tablets with Various Excipients (After stability test)
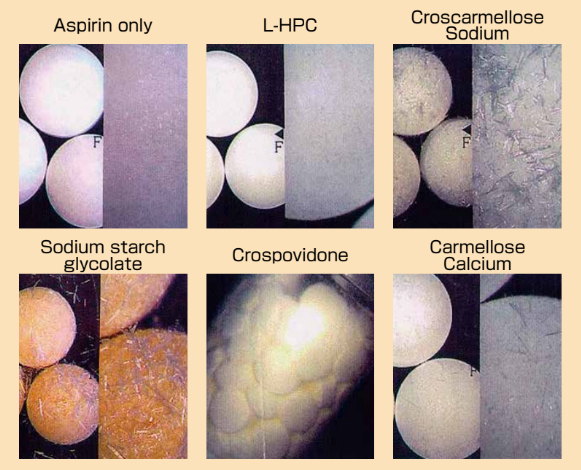
Aspirin tablets with 20% excipients were stored in closed plastic bottle at 50°C for 3 months.
Packaging & Availability
Storage & Handling
- Storage
Keep dry. Store away from excess heat and sunlight. Store in sealed containers.