Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- INCI Name
- Ingredient Origin
- Cosmetic Ingredients Functions
- Technologies
- Product Families
Features & Benefits
- Benefit Claims
- Labeling Claims
- Product Highlights
- High absorption capacity (BET surface area 400–450 m2/g)
- Irregular shaped particles
- Simultaneous absorption of hydrophilic and lipophilic substances
- Increased adhesion to skin
- Absorption inside porous (pore volume 0.40–0.50 cm3/g)
- Viscosity modification
- Light scattering due to amorphous and porous structure, and irregular shaped particles
Applications & Uses
- Markets
- Applications
- Application Format
- AP/Deo Applications
- Color Cosmetic Applications
- Hair Care Applications
- Skin Care Applications
- Treatment Product Applications
Properties
- Physical Form
- Appearance
- Fine white powder
- Odor
- Slightly characteristic
- Microbiological Values
Value Units Test Method / Conditions Aerobic bacteria max. 10 CFU/g - Moulds max. 10 CFU/g - Yeasts max. 10 CFU/g - Total Aerobic Microbial Count (TAMC) max. 10 CFU/g - Total Combined Yeasts and Moulds Count (TYMC) max. 10 CFU/g - - Specifications
Value Units Test Method / Conditions Bulk density (at 20°C) 200 - 400 kg/m³ - Oil uptake 1.0 - 1.3 g oil/g powder Particle size (Dᵥ50) 2 - 10 μm BET Surface Area 400 - 450 m²/g Pore Volume 0.40 - 0.50 cm³/g - Impurities
Value Units Test Method / Conditions Volatile Organic Compound* (Methanol)** max. 3 % GC - Heavy Metals
Value Units Test Method / Conditions Antimony Content max. 0.5 mg/kg ICP-MS Arsenic Content max. 0.5 mg/kg ICP-MS Lead Content max. 0.5 mg/kg ICP-MS Cobalt Content max. 3.0 mg/kg ICP-MS Chromium Content max. 10.0 mg/kg ICP-MS Nickel Content max. 5.0 mg/kg ICP-MS Cadmium Content max. 0.1 mg/kg ICP-MS Mercury Content max. 0.1 mg/kg ICP-MS - Note
* Methanol is considered to be a VOC under normal conditions. However, methanol is bound in Upsalite® to the degree that it does not fall into the definitions of a VOC.
** Methanol is a residue from the synthesis process thus, the presence in the material is non-intended and technically unavoidable.
Regulatory & Compliance
Technical Details & Test Data
- Physical Characteristics
- Cosmetic grade Upsalite® (Figure 1) is a fine white powder consisting of amorphous, anhydrous and porous magnesium carbonate with residuals of crystalline magnesium oxide.

Figure 1: Cosmetic grade Upsalite powder
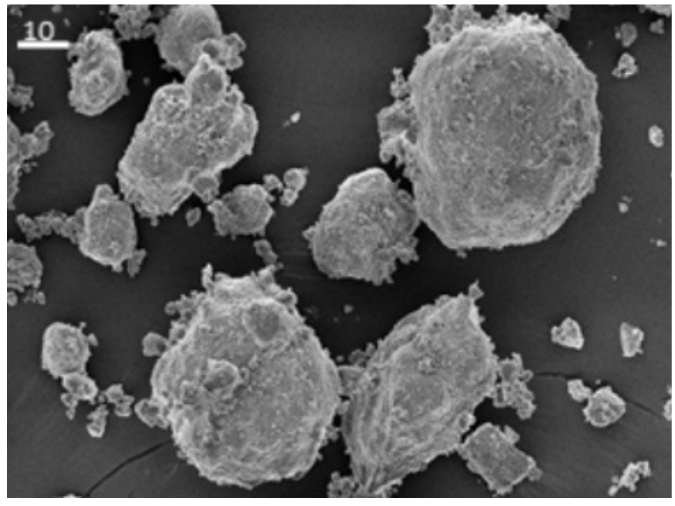
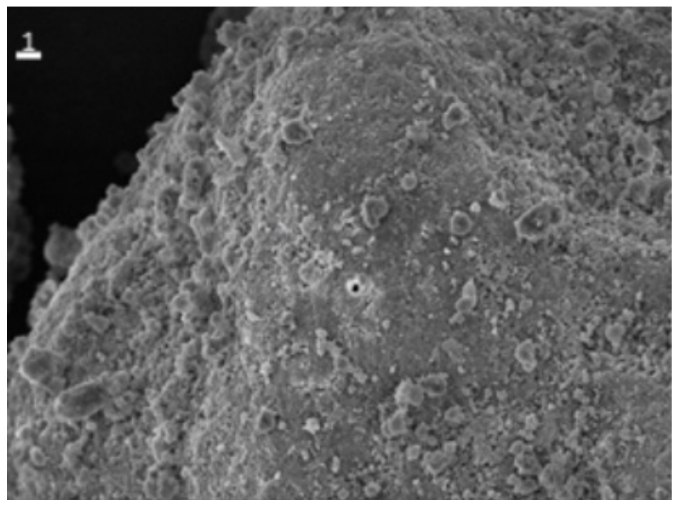
Figure 2 : Scanning electron microscopic (SEM) images of Upsalite showing different size irregular shaped par ticles (left) and the close-up structure (right) of Upsalite.
- Upsalite is a porous material with a narrow peak pore size distribution in the lower range of the mesoporous scale (2–6 nm).
- Cosmetic grade Upsalite is milled to a fine powder with a Dv50 particle size around 5 μm (Figure 3) and has a large pore volume, from 0.4 to 0.6 cm3/g and a surface area (BET) of at least 150 m2/g (Figure 4).
- Cosmetic grade Upsalite is not a nanomaterial but contains numerous nano-sized pores resulting in a unique absorption capacity.
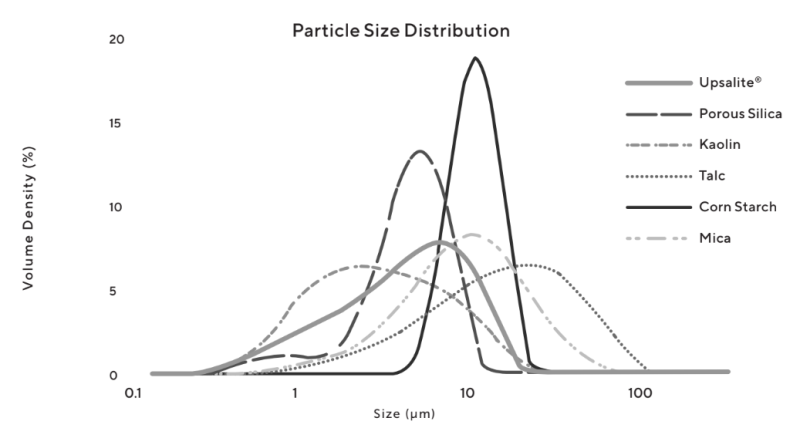
Figure 3 : Graph showing the par ticle size distribution of Cosmetic grade Upsalite as compared to other cosmetic powders .
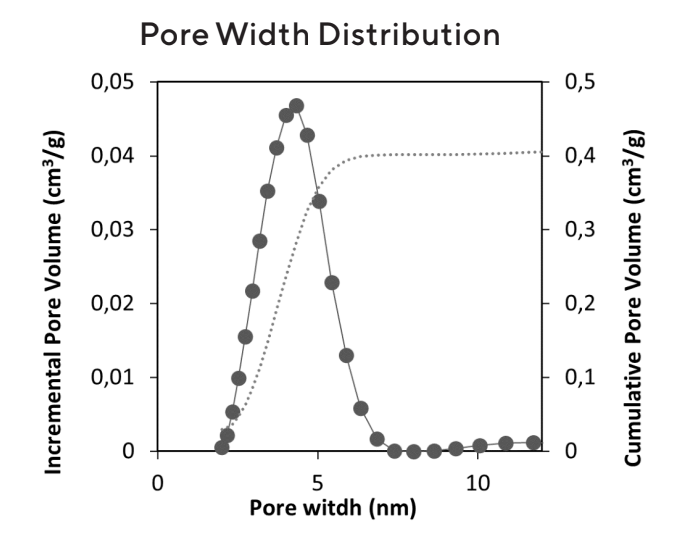
Figure 4: Typical pore width distribution and cumulative pore volume of Upsalite C101 grade. Cosmetic grade Upsalite is not a nanomaterial but contains numerous nano sized pores.
- Oil and Water Absorption
- Upsalite can take up more than 1 g oil per g material and, in contrast to many other oil-absorbing materials that bind oil on the surface, Upsalite is absorbing the oil within the pores, thereby maintaining the dry and non-greasy feeling, even after oil uptake (Figure 5 and 6).

Figure 5 : Photographs of different powders after oil uptake.
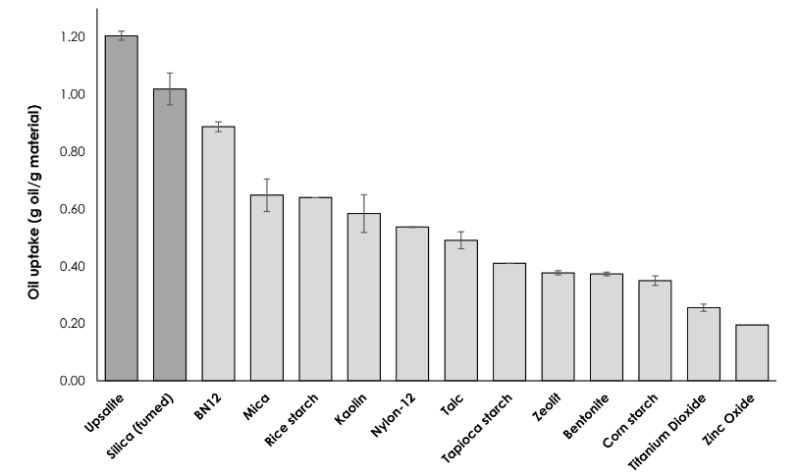
Figure 6 : Oil uptake of Upsalite as compared to other absorbing powders.
- This gives the particles the dual effect of both hiding the oil inside the structure while also creating the surface needed for a matte appearance (Figure 7).
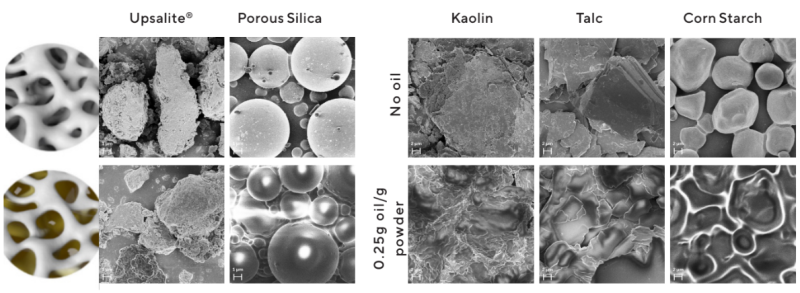
Figure 7 : Schematic illustrations and SEM images of Upsalite, porous silica, kaolin, talc and corn starch, before (top), and after (bottom) oil uptake. Note the dry separated particles of Upsalite even after 0.75 g/g uptake as compared to the oily appearance of the other cosmetic powders after oil uptake.
- In contrast to many other porous materials, Upsalite can absorb both oil and water without any additional modification or surface treatment and has the ability to take up water even if it has absorbed oil and vice versa (Figure 8).
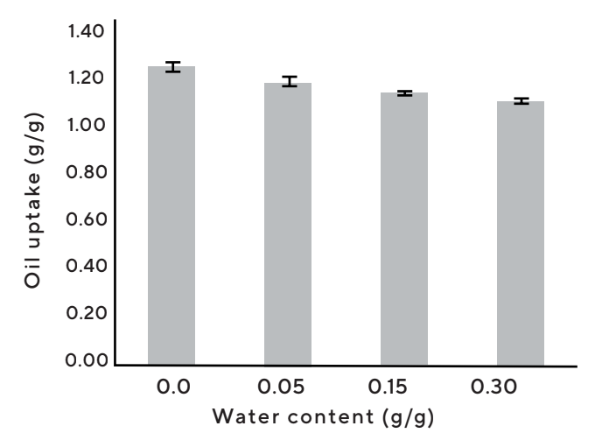
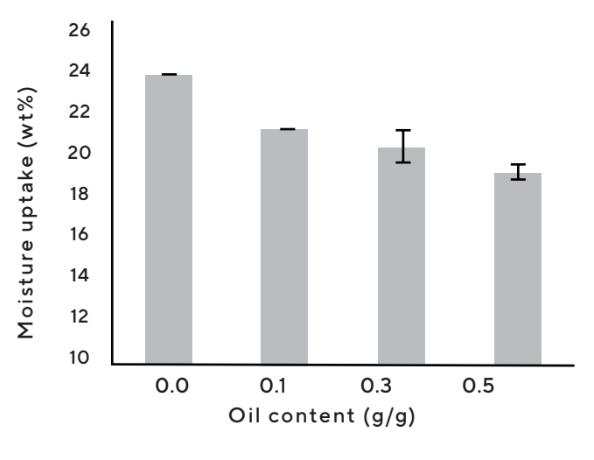
Figure 8 : Cosmetic grade Upsalite maintains the capacity to take up oil even af ter water uptake and can also absorb moisture when loaded with up to 0.5 g oil/g powder. Note that wt% moisture absorption equals to % moisture of total weight.
- This capacity is an advantage since Upsalite can absorb both excess oil (sebum) and water (perspiration) without drying out the skin.

Figure 9 : Absorption Speed test of commonly used ingredients
- Dermatologically Tested
Upsalite® is dermatologically tested. The viability of Reconstructed human epidermal model was > 50%, according to results obtained from in vitro EpiDermTM Skin Irritation Test (EPI-200- SIT). Thus, Upsalite® is classified as: NI (Not Irritant)/not required classification (External study performed under GLP and GCP at QACS Ltd laboratory).
A human repeated insult patch test (HRIPT) on 50 volunteers showed no dermal sensitization potential of 100% Upsalite® applied topically on the skin. None (0%) of the 50 volunteers exhibited any irritant reaction, erythema, dryness or oedema; neither before the first application, during the induction period, nor after the challenge phase according to the scale used for the interpretation of the results (external results from QACS Ltd).
Storage & Handling
- Shelf Life
- min. 24 months
- Storage and Stability
- Upsalite® maintains its properties with storage in a fully sealed and dry container. It can be formulated with maintained integrity in solvents such as ethanol or in other anhydrous formulations.
- It is important to avoid contact with acids as Upsalite® will dissolve at low pH.
- Upsalite® thermally decomposes to magnesium oxide and CO2 above 320°C.
- Shelf Life and Storage Conditions
Upsalite® is a hygroscopic material and adsorbs moisture from the atmosphere. Prompt, airtight closing of the packaging after usage is therefore important. Shelf life is min. 24 months when stored in a dry environment, below 25°C (unopened original packaging).