Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Chemical Family
- Ingredient Name
- Food Ingredients Functions
- Plastics & Elastomers Functions
- Food Additive Number
- E 900a, INS 900 a
- Ingredients
- Dichlorodimethylsilane, Chlorotrimethylsilane
- Technologies
- Product Families
- Chemical Structure
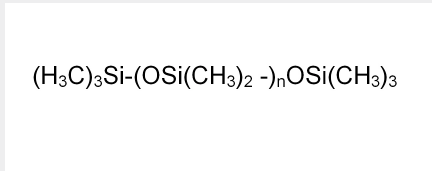
Features & Benefits
- Labeling Claims
- Food Ingredients Features
- Materials Features
Applications & Uses
- Markets
- Applications
- Food & Nutrition Applications
- Plastics & Elastomers End Uses
- Application Details
WACKER® FG 350 is used predominantly to control foam in lipophilic systems (foods rich in oil or fat, example deep-frying oil) or for surface treatment. WACKER® FG 350 also serves as the base material in the production of antifoams compliant with the regulations pertaining to food for human consumption.
Properties
- Color
- Physical Form
- Odor
- Odorless
- Typical Properties
Value Units Test Method / Conditions Acidity max. 5 mg/kg Direct Titration Method Active Substance 100 % - Refractive Index 1.402 - 1.404 - - Solidification Point -50 °C -
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Quality Standards
- Regulatory Status
Direct Food additive:
- WACKER® FG 350 conforms to FDA 21 CFR § 173.340 "Defoaming Agents" as a Secondary Direct Food Additive Permitted in Food for Human Consumption." However, no Food Safety Modernization Act (FSMA) compliant program is implemented for this product.
- WACKER FG 350 is listed in the Regulation (EC) No 1333/2008 Annex II and III as amended by Regulations (EU) Nos 1129/2011 and 1130/2011 as E 900 polydimethylsiloxane. It meets the purity requirements as laid down in Regulation (EU) No. 231/2012 and is therefore approved as direct food additive within the EC under observance of the use restrictions in force for polydimethylsiloxane (E 900).
- WACKER FG 350 is certified according to HACCP principles. Hence, it meets the requirements on food hygiene in accordance with Regulation (EC) No. 852/2004.
Food Contact:
- WACKER FG 350 complies with the Recommendation "XV Silicones" of the BfR concerning the manufacture of essential commodities that have contact with food.
- WACKER® FG 350 is listed under PM-Nr. 76721 in Regulation (EU) No. 10/2011 as an additive for plastic materials to come into contact with food and meets the specifications under column (4) and (10). The product is not subject to a specific migration limit (SML).
WACKER® FG 350 meets the criteria for an exemption from the requirements of a tolerance when used as an additive in pesticide formulations to be applied to growing crops or raw agricultural commodities after harvest as specified in 40 CR 180.960.
National regulations applicable in the countries concerned must be observed.
Packaging & Availability
- Country Availability
- Regional Availability

