Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
- CAS No.
- 9004-65-3
- EC No.
- 618-389-6
- Technologies
- Product Families
Features & Benefits
- Labeling Claims
- High Quality
Manufactured in Germany, Tylopur Xtend Nutra ® is a plant derived product that complies to European and US guidelines and guarantees exclusion of non-conforming ingredients (dioxides, stearates).
Applications & Uses
- Markets
- Applications
- Dosage Form
- Manufacturing Technology
- How it Works
Release Profile of Typical Nutraceutical Compounds:
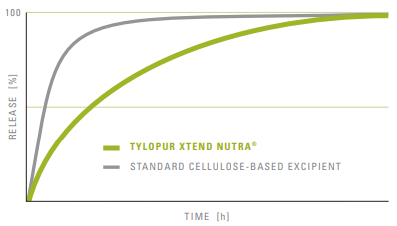
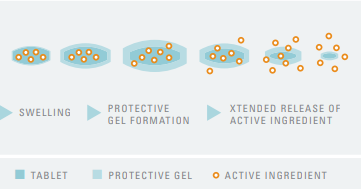
Fig: Functionality of Tylopur Xtend Nutra®
Properties
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Grade
Packaging & Availability
- Packaging Type
- Packaging
The carton box packaging consists of an inner polyethylene (PE) bag and an outer carton box. The inner PE bag is sealed with an irreversible plastic tie. The outer carton box is sealed with a tamper proof, adhesive tape. Each component of the Carton box packaging is new material, not recycled.
Storage & Handling
- Shelf Life
- 3 years
- Expiration Date and/or Recommended Re-Evaluation Interval
Re-evaluation date:
- The date after 3 years from the manufacturing date.
- If used after the re-evaluation date, it is recommended to analyze the quality of Tylopur® before use.
- Storage and Shipping Conditions
Keep dry. Store in sealed original packaging and away from excess heat and sunlight.

