Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
- CAS No.
- 9004-65-3
- EC No.
- 618-389-6
- Technologies
- Product Families
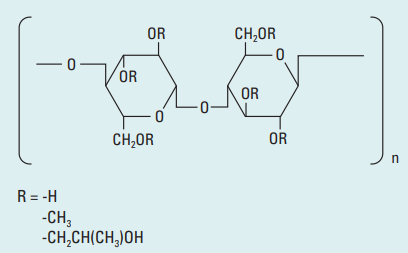
- Structure
Features & Benefits
- HPMC Key Quality Attributes in Matrix Tablets
- Decrease in initial erosion
- Faster drug release
- No effect on tablet hardness
Applications & Uses
- Markets
- Applications
- Dosage Form
- Manufacturing Technology
- Product Applications
- Tablet/ pellet film coating
- Granulation binder
- Suitable for capsule shell
- Suitable for dry syrup
- Suitable for oral strips
Properties
- Physical Form
- Appearance
- Conforms
- Typical Properties
Value Units Test Method / Conditions Heavy Metals max. 20 ppm EP Viscosity 3 - 15 mPa.s - Viscosity 4.0 - 6.0 mPa.s EP HPMC 6.75 %w/w - Hydroxypropoxy Content 7.0 - 12.0 % EP Loss on Drying max. 5.0 % EP Talc 2.85 %w/w - Methoxy Content 28.0 - 30.0 % EP TiO₂ 3.9 %w/w - pH 5.0 - 8.0 - EP Pigment 0.15 %w/w - PEG 6000 1.35 %w/w - Water 85 %w/w - Solid Content 15 %w/w - Nozzle Type Schlick 970 - - Nozzle Pressure 0.65 bar - Inlet Air Flow (Coating Parameters) constant m³/h - Inlet Air Flow (Coating Parameters) 80 °C - Tablet bed temperature pre-heating (Coating Parameters) 40 °C - Tablet bed temperature during coating 38 - 40 °C - Spray Rate (coating parameters) 4.5 - 6.4 g/min - Pan speed (Coating Parameters) 22 rpm - Weight 258.8 mg - Disintegration time 559 sec - Dissolution (>80 %) 10 min - Identification (A - E) Conforms - EP Sulphated Ash/ Residue on Ignition max. 1.5 - EP - Note
- Harmonized items among the USP, EP and JP
- Specific local attribute in EP
- Viscosity is measured with 2 % aqueous solution at 20 °C. Viscosity ranges are 80 % – 120 % of the nominal value for samples with a viscosity less than 600 mPa.s and 75 % – 140 % of the nominal value for samples with 600 mPa.s or higher. Viscosity is hormonized item among the USP, EP and JP.
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Grade
- Regulatory Information
- EU-Regulations
- No REACH Annex XVII restrictions. This product is not on the REACH Candidate List
- This product is not on the REACH Annex XIV List
- This product Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH) with its amendment Regulation (EU) 2020/878 SDS No: 10896-0310 16/03/2021 (Revision date) GB - en 8/9
- This product is not subject to Regulation (EU) No 649/2012 of the European Parliament and of the Council of 4 july 2012 concerning the export and import of hazardous chemicals. This product is not subject to Regulation (EU) No 2019/1021 of the European Parliament and of the Council of 20 June 2019 on persistent organic pollutants
- Contains no substance subject to Regulation (EU) 2019/1148 of the European Parliament and of the Council of 20 June 2019 on the marketing and use of explosives precursors.
- National regulations
- Listed on the United States TSCA (Toxic Substances Control Act) inventory
- Listed on the Canadian DSL (Domestic Substances List)
- Listed introduction on Australian Industrial Chemicals Introduction Scheme (AICIS Inventory)
- Listed on the EEC inventory EINECS (European Inventory of Existing Commercial Chemical Substances)- Directive 79/831/EEC, sixth Amendment of Directive 67/548/EEC (dangerous substances)
- Listed on the Japanese ENCS (Existing & New Chemical Substances) inventory Listed on PICCS (Philippines Inventory of Chemicals and Chemical Substances)
- Listed on NZIoC (New Zealand Inventory of Chemicals)
- Listed on the TCSI (Taiwan Chemical Substance Inventory) Listed on IECSC (Inventory of Existing Chemical Substances Produced or Imported in China) Listed on KECL/KECI (Korean Existing Chemicals Inventory)
Packaging & Availability
Storage & Handling
- Storage Information
- Keep dry. Store away from excess heat and sunlight.
- Store preferentially in original packaging.

