Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
- CAS No.
- 9004-65-3
- EC No.
- 618-389-6
- Technologies
- Product Families
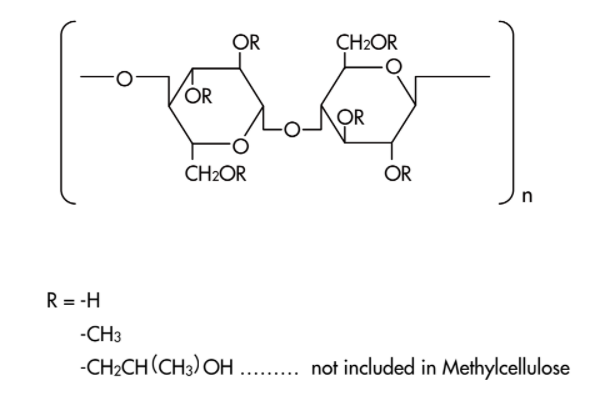
- Structure
Features & Benefits
- Labeling Claims
- Features
- For Hydrophilic matrix formulation of API and METOLOSE® SR to extend the dissolution. The dosage forms can be obtained by direct compression or granulation process.
- METOLOSE® SR has specifications of particle size which can be suitable for sustained release application.
- Dissolution profile can be easily adjusted by selecting appropriate grade.
- Recommendable amount of METOLOSE® SR is more than 20% in the formulation in order to form the stable gel layer.
Applications & Uses
- Markets
- Applications
- Dosage Form
- Manufacturing Technology
Properties
- Physical Form
- Typical Properties
Value Units Test Method / Conditions Hydroxypropoxy Content 8.5 - 10.5 % - Methoxy Content 22.0 - 24.0 % - Viscosity 75000 - 140000 - - - Particle Size Distribution
Value Units Test Method / Conditions Particle Size at D20 20 - 40 µm - Particle Size at D50 50 - 80 µm - Particle Size at D80 100 – 160 µm - Note
*Values in the table are viscosities of 2 w/w % aqueous solution at 20°C
Regulatory & Compliance
Technical Details & Test Data
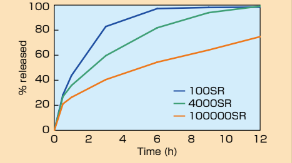
- Drug Release from Matrix Tablets with Metolose® SR (Viscosity Grade)
Packaging & Availability
- Packaging Type
- Package
- Packaging material: Double-layered polyethylene bag in fiber drum
- Net weight: Depending on grades
Storage & Handling
- Storage
Keep dry. Store away from excess heat and sunlight. Store in sealed containers.