Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Chemical Name
- Ingredient Name
- Animal Feed & Nutrition Functions
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
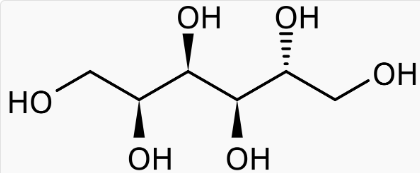
- Chemical Structure
Features & Benefits
- Labeling Claims
- Food Ingredients Features
Applications & Uses
- Markets
- Applications
- Dosage Form
- Product Applications
- NEOSORB® 70/70 B is a non-crystallizing liquid sorbitol. It is used as a plasticizer in soft gelatin capsules, as a bulk sweetener in liquid dosage forms or as a humectant in semi-solids.
- NEOSORB® 70/70 B contains sorbitol and other hydrogenated oligomers.
- It can be used as substitute for sucrose and meet organoleptic (natural sweetness) and health requirements (it is sugar-free, low-calorie, does not induce dental caries, and may be advised for diabetics).
- NEOSORB® 70/70 B is available as non-GMO.
Properties
- Physical Form
- Soluble In
- Appearance
- syrupy liquid, clear colourless
- Taste
- Sweet
- Odor
- Sweet
- Miscible In
- Water
- Insoluble in
- Vegetable oils, Mineral oils
- Typical Properties
Value Units Test Method / Conditions Average Molecular Weight (D-Sorbitol) 182.2 g/mol Water Content (Loss on Drying) 28 - 32 % European Pharmacopoeia - Microbiological Values
Value Units Test Method / Conditions Total Aerobic Microbial Count max. 1000 CFU/g National Formulary Yeasts and Moulds Count max. 100 CFU/g National Formulary Escherichia coli(**) 0 /g - Salmonella(**) 0 /10g - - Composition
Value Units Test Method / Conditions D-Sorbitol Content (on D.S) 72.0 - 92.0 % European Pharmacopoeia - Specifications
Value Units Test Method / Conditions Reducing Sugars max. 0.2 % European Pharmacopoeia Reducing Sugars (after hydrolysis) max. 9.3 % European Pharmacopoeia Dry Substance 68.0 - 72.0 % European Pharmacopoeia D-sorbitol Content (as is) min. 45.0 % National Formulary Residue on Ignition(*) max. 0.1 % National Formulary Nickel Content max. 1 mg/kg National Formulary Reducing Sugars (on DS) max. 0.3 % National Formulary pH 5.0 - 7.5 - National Formulary Water Content 28.5 - 31.5 % National Formulary Conductivity max. 10 microS/cm European Pharmacopoeia - Note
(*) Compliance data - Tests not performed
(**) Monitoring plan
Regulatory & Compliance
Technical Details & Test Data
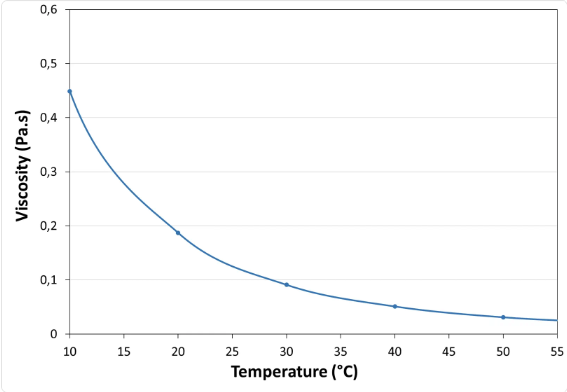
- Viscosity vs Temperature Profile
Packaging & Availability
- Packaging Information
- Article (SKU) Code: 5100000823
- Package Size & Type: 275 KG LIQUID DRUM
Storage & Handling
- Shelf Life
- 3 years
- Storage and Shelf Life Information
- Shelf Life of this product is manufacturing date + 3 years, in its unopened packaging.
- The product durability may vary according to packaging type and manufacturing plant.
- We recommend to preserve the product in its unopened original packaging, preferably protected from wide variations of temperature and humidity.
- Upon opening, use the product as quickly as possible to prevent moisture regain.