Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
- CAS No.
- 9063-38-1
- EC No.
- 618-597-7
- Technologies
- Product Families
Features & Benefits
- Labeling Claims
- Benefits of Superdisintegrants
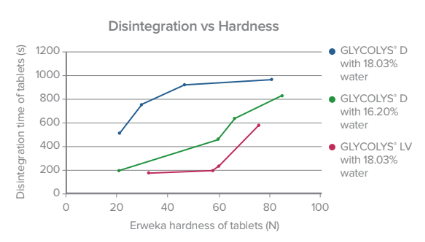
Our superdisintegrant offerings meet the requirements necessary to achieve fast and robust disintegration with minimal amount of disintegrating agent. These properties facilitate the use of any ingredient you need in order to optimize other parameters of your formulation (compactability or drug solubility for example).
GLYCOLYS®, one of our sodium starch glycolate (SSG)-based products, offers solutions to specific environments and conditions susceptible to affect disintegrant performance as well as ensuring stability for drugs, like GLYCOLYS® Low pH ensures stability to acidic molecules.
Applications & Uses
- Markets
- Applications
- Dosage Form
- Manufacturing Technology
- Use Level
- 2 - 8%
Properties
- Physical Form
- Appearance
- White fine powder
- Insoluble in
- Methylene Chloride
- Typical Properties
Value Units Test Method / Conditions Combined Sodium Content 2.8 - 4.2 % Ph.Eur.-USP-JP Ethanol Content max. 6 % Ph.Eur.-USP Heavy Metals Content max. 20 mg/kg JP Iron Content max. 20 mg/kg Ph.Eur.-USP-JP Loss on Drying max. 10 % Ph.Eur.-USP-JP Particle Size (Retained on 200 mesh. 75 microns) max. 10.0 % - pH max. 10.0 - - Sodium Chloride Content max. 7.0 % Ph.Eur.-USP-JP Sodium Glycolate max. 2.0 % Ph.Eur.-USP-JP Water Content max. 10 % - Microbiological Values
Value Units Test Method / Conditions Escherichia coli Not Detected per gram - Pseudomonas aeruginosa Not detected per gram - Salmonella Not Detected per 10g - Staphylococcus aureus Not detected per gram - Total Aerobic Microbial Count max. 100 CFU/g - Total Yeasts and Moulds Count max. 10 CFU/g -
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Grade
- Quality Standards
- Conformity
Complies with the current USP/NF,EP and JP monographs requirements
Technical Details & Test Data
- Innovation Hub
- Innovation Hub by RoquetteLooking for technical support or formulation inspiration? Visit Roquette’s Innovation Hub.
Packaging & Availability
- Packaging Information
- Article (SKU) Code: 5100003101
- Package Size & Type: 30 KG CARBOARD BOX
Storage & Handling
- Shelf Life
- 5 Years
- Storage and Handling
Storage in well-closed containers preferably protected from wide variations in temperature and humidity, which may cause caking