Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- INCI Name
- Cosmetic Ingredients Functions
- Molecular formula
- C₂₂H₃₀Cl₂N₁₀.2C₆H₁₂O₇
- Technologies
- Product Families
- Identification
- IR Spectrum (sample should correspond with that of CRS)
- Thin layer Chromatography (the principal spot should correspond with the reference solution)
- Residual melting Point: 132 C / Chemical Test: deep red color should be produced
- Structure
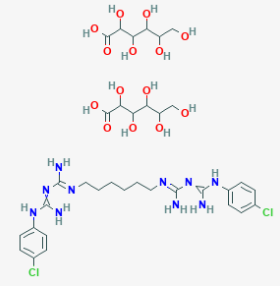
Features & Benefits
- Benefit Claims
- Product Highlights
- Chlorhexidine Gluconate 20% Solution is a broad spectrum bacteriostatis antiseptic agent, oral care agent, disinfectant, cosmetic biocide, and preservative.
- Chlorhexidine Gluconate 20% is very effective against plaque, oral flora including Candida and is active against gram-positive and gram- negative organisms, facultative anaerobes, aerobes, and yeast. It can be used in antiseptic soap, mouthwash that fights plaque, disinfecting wounds and burns, vaginal flushing, hair dyes and bleaches, makeup, and other skin and hair care products.
- Chlorhexidine gluconate 20% matches the chemical standards of the industry.
- Local Anti-infectives, Miscellaneous
Applications & Uses
- Markets
- Applications
- Application Format
- Bath & Shower Applications
- Hair Care Applications
- Oral Care Applications
- Personal Hygiene Applications
- Skin Care Applications
- Treatment Product Applications
- Uses
- Chlorhexidine gluconate 20% is an high quality antiseptic used in a broad field of indications.
- Definition of Antiseptic (Anti-Infective Agents, Local) - Substances used on humans and animals that destroy harmful microorganisms or inhibit their activity.
Topical use
- Surgical scrub and antiseptic hand rinse for healthcare personnel
- Skin cleanser for preoperative skin preparation, skin wound and general skin cleanser for patients
Oral use
- Oral rinse: 0.12% Antibacterial dental rinse for gingivitis treatment
- Periodontal chip: Adjunctive therapy to reduce pocket depth in patients with periodontitis
Veterinary use
- Effective protection against Mastitis by cows
- Used in the general dairy hygiene of milk producing animals
Properties
- Physical Form
- Typical Properties
Value Units Test Method / Conditions Concentration (Chlorhexidine Di Gluconate) 0.5 - 4 % - pH (5%V/V Solution) 5.5 - 7.0 - - Relative Density 1.06 - 1.07 - - Assay (By Potentiometricaly) 190 - 210 g/L - Molecular Mass 897.762 g/mol - - Solubility
Miscible with water, with not more than 3 parts of acetone and with not more than 5 parts of ethanol (96%).
- Microbial Purity
Chlorhexidine gluconate 20% is microbial free.
- Microbials are extremely resistant to even the harshest and most unlivable conditions.
- The so called extremophiles (extremophilic microbials) live in habitats which for other terrestrial life-forms are intolerably hostile or even lethal. Some live in extreme hot niches, ice, and salt solutions, as well as in acid and alkaline conditions. Some may grow in toxic waste, organic solvents, heavy metals, or in several other habitats that were previously considered inhospitable for life.
- So it is not surprisingly, that even in a medium that is used as a disinfectant, microbials can life and grow.
- RN Laboratories is aware of those specific microbials. Contamination is prevented by
- own in house production in dedicated plants under controlled atmosphere
- strictly use of purified water only
- release of all batches only after a validated microbial test.
Regulatory & Compliance
- Certifications & Compliance
- Specification At Release
EP/BP/USP as per requirement.
- Regulatory Status
Chlorhexidine gluconate 20% matches all the regulatory standards with a cep covering 2 production sites, the echa authorization to sell for biocidal use under art 95 and a reach registration.
API (Ph. Eur.)
- CEP (Certificate of suitability of monographs of the European Pharmacopeia) issued R1-CEP 2006-171-REV 02
- EU-GMP (Good Manufacturing Practice)
- DMF (Drug Master File) issued 2006 No.21946
Biocidal use (Art 95)
- RN Laboratories is allowed to sell chlorhexidine digluconate as a biocidal active under Art 95 through an approved letter of access to the original dossier of Evonik/Medichem for the following product types:
- Product type 1: Human hygiene biocidal products
- Product type 2: Private area and health are disinfectants
- Product type 3: Veterinary hygiene biocidal products
Cosmetic
- Chlorhexidine digluconate is registered under REACH No. 01-2119946568-22-0005
- Chlorhexidine digluconate is registered in the CosIng (Cosmetic Ingredient Database). This means that it can be used in cosmetics as a preservative with a maximum concentration of 0.3%.
Packaging & Availability
- Packaging Type
- Availability
Chlorhexidine Gluconate is mainly available in OTC products to clean and prepare the skin before surgery and before injections in order to help reduce bacteria that potentially can cause skin infections. These products are available as solutions, washes, sponges, and swabs and under many different brand names.
- Packaging
IBC’s 1000kg, 200kg drums, 50kg drums, 25 kg drums.
Storage & Handling
- Stability
- 36 months
- Storage Conditions
- Store below 25C
- Keep the original drums tightly closed
- Keep away from direct sunlight
- Do not freeze