Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Chemical Family
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Applications & Uses
- Markets
- Applications
- Dosage Form
- Use application
For tablet, capsule, granule and other general foods.
Properties
Regulatory & Compliance
- Certifications & Compliance
- Grade
- Complied
Fully complied with EU food regulation.
Technical Details & Test Data
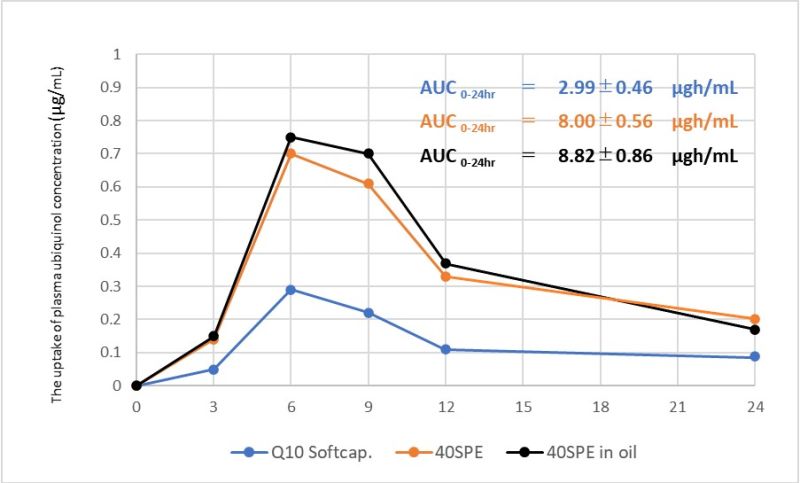
- The bioavailability of Water-dispersive Powder (40SPE) after single oral administration
In this study, we showed the bioavailability of Coenzyme Q10 40% Water-dispersive Powder-E (40SPE). 4 healthy subjects received single oral administration of 100mg of Q10 in the form of a soft capsule※ containing Q10 dissolved in Olive oil, a 40SPE in a hard capsule and a 40SPE mixed with Olive oil in a hard capsule in the fasting period, and changes in the plasma ubiquinol concentration were monitored over time. AUC0-24hr values were 2.99, 8.00 and 8.82μgh/ml, respectively. 4 healthy subjects did not take any coenzyme supplements or drinks for 3 weeks, and did not take anything but water from 6:00 p.m. on the day before to 6:00 p.m. on the day of trial. The same 4 subjects repeated the trial in the same manner.
※Ingredients of commercial soft capsule:CoQ10, Olive oil, glycerin, lecithin, vitamin E, etc.