Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Chemical Family
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Applications & Uses
- Markets
- Applications
- Dosage Form
- Use application
For beverage (water-based drinks)
Properties
Regulatory & Compliance
- Certifications & Compliance
- Grade
Technical Details & Test Data
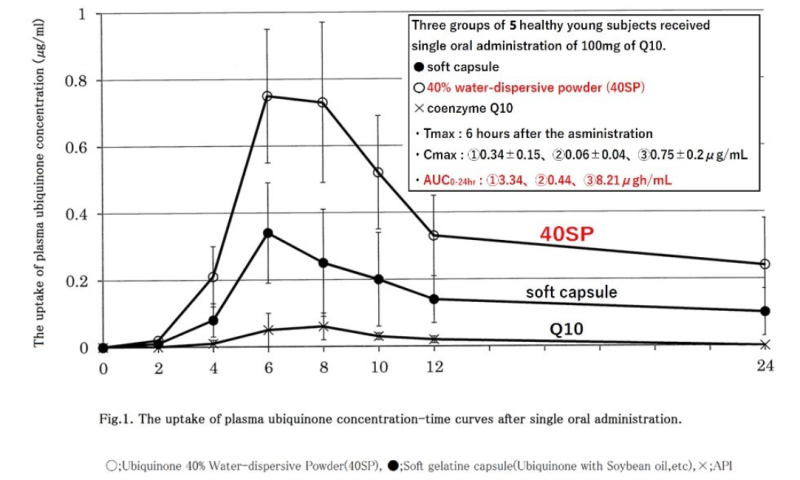
- The bioavailability of coenzyme Q10 Water-dispersive Powder (40SP) after oral administration
In this study, we showed the bioavailability of ubiquinone (Q10) in the form of water-dispersive Powder. Three groups of 5 healthy young subjects received single oral administration of 100mg of Q10 in the form of a soft capsule containing Q10 dissolved in soybean oil, API or 40% Water-dispersive Powder (40SP) in the fasting period, and changes in the plasma Q10 concentration were monitored over time. Tmax was 6 hours after the administration. Cmax values compared with the pre-administration baseline in the soft capsule, API and 40SP groups were 0.34±0.15, 0.06±0.04 and 0.75±0.2μg/ml, respectively. AUC0-24hr values were 3.34, 0.44 and 8.21μg・h/ml, respectively. The three types of Q10 preparations that were compared in this study showed clear differences when they were administered once under fasting conditions; 40SP showed better results. A customary soft capsule that contains Q10 dissolved in lipids can be absorbed well when taken in conjunction with meals. However, it was found that 40SPcoukd be absorbed whenever it was taken. 40SP can be processed into various forms, such as hard capsules, tablets and powder. Furthermore, when dissolved in water, particles are dispersed stably with the average particle radius of 100nm (nanometre), and thus, 40SP may also be applocable to drinks. 40SP can be prepared in various forms, is anticipated to find new applications in supplements and drinks.