Enhanced TDS
Knowde-enriched technical product data sheet
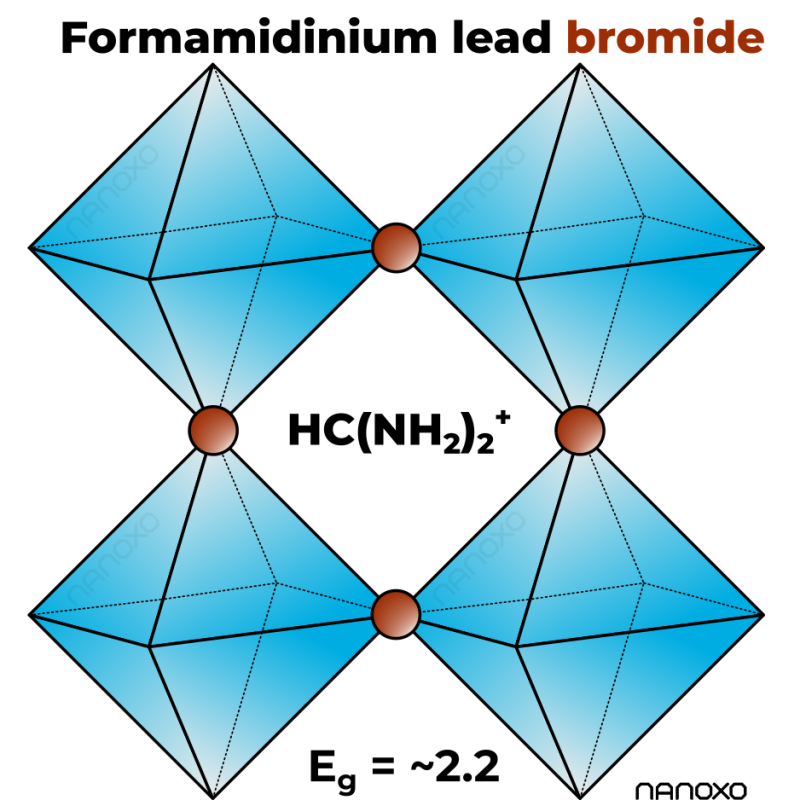
Identification & Functionality
- Chemical Family
- Chemical Name
- Technologies
- Product Families
Features & Benefits
- Base Chemicals Features
Applications & Uses
- Markets
- Applications
- Base Chemicals End Uses
- Perovskite Ink Solution Preparation
- In order to prepare perovskite inks, it is recommended to use the original amber glass vessel filled with argon gas.
- Inks can be prepared by dissolving perovskite in organic polar aprotic solvents at mild temperatures of 60-80°C. To acquire a solution with a high concentration Cn >1 mol/dm³, use gamma-butyrolactone (GBL) or a mixture of dimethylformamide (DMF) and dimethylsulfoxide (DMSO) in a 4:1 volume ratio. Use the following molar masses for concentration calculations, being aware of volume expansion phenomena for low volume solutions.
Perovskite Molar mass (g/mol)β-MAPbl₃ 619.98 α-FAPbl₃ 632.98 y-CSPbl₃ 720.82 GuaPbl₃ 64799 α-MAPbBr₃ 478.98 α-FAPbBr₃ 491.98 y-CSPbl₃ 579.82 - The dissolution process might be enhanced with the use of an ultrasonic bath or magnetic stirring.
- For the best quality of perovskite inks, use anhydrous solvents only. After preparation, the solution should be used within 24 hours period to avoid secondary chemical reactions leading to product decomposition.
- Store ink solution in a dark and dry place, preferably in a glove box or under inert gas at room temperature.
Properties
- Physical Form
- Appearance
- Orange powder
- Soluble in
- Polar aprotic solvents like DMF, DMSO, GBL
- Typical Properties
Value Units Test Method / Conditions Purity 99.9 % Trace metal basis* Band Gap approx. 2.2 eV - - Physico-Chemical Properties
Value Units Test Method / Conditions Molecular Weight 491.98 g/mol - - Note
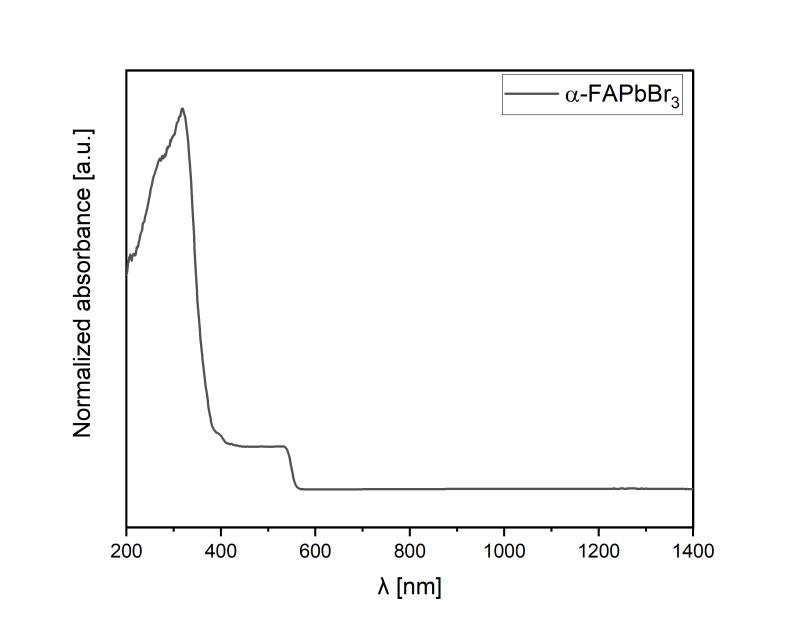
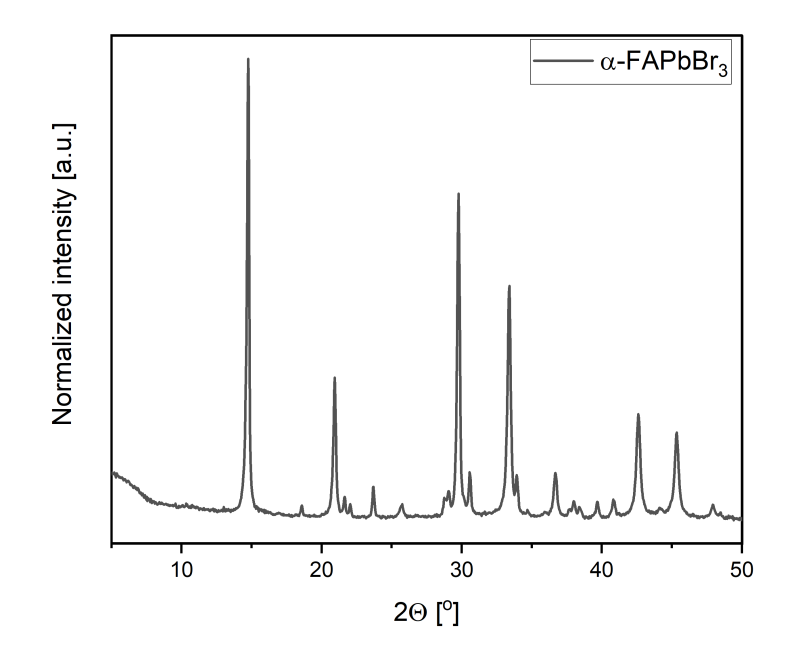
*The quality and phase-purity of the final product is determined with PXRD and UV-VIS-NIR techniques.
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- Technical Details
Packaging & Availability
- Packaging Information
Catalog Number Quantity FAPbBr₃-S-500 500mg FAPbBr₃-S-2 2g
Storage & Handling
- Shelf Life
- 6 months
- Storage Conditions
- Suitable for room temperature storage
- Store under inert gas in dark
- Hygroscopic
- Basic Treatment & Handling
- Perovskites are delivered in air-tight vials of amber glass vessels, equipped with a double-sided silicone/PTFE septum. Products are synthesized, packed, and stored under an inert argon atmosphere.
- To preserve the quality of the perovskites, avoid direct contact with oxidizing or reducing agents. Keep the products in the dark under an inert gas atmosphere. preferably in a glove box. To manipulate products, use glove-box or Schlenk line (air-free technique).
- Due to the acute toxicity of lead halide perovskites, avoid skin contact, long-time dust exposure, and oral ingestion.
- Due to intrinsic phase metastability, some selected products may undergo phase transition or segregation back to δ-perovskite yellow phases. Before use, please consider reheating the metastable products, to achieve complete conversion to the desired α- or y- perovskites polymorphs (i.e., FAPbl₃ and CSPbl₃). FAPbl₃ should be heated up to 150°C, while CSPbl₃ up to 325°C. Therefore, in the case of the presence of δ- perovskite yellow phases, heat the samples with respective temperatures. Visual assessment may be used to indicate conversion rate. After the color change to black, heat the powders for the next 15-30 minutes. The preferable methods for following conversion rates are powder X-ray diffraction technique (PXRD) and ultraviolet and visible light spectroscopy (UV-VIS). To avoid thermal decomposition, do not overheat the products.
- Good laboratory practice includes lead waste management in agreement with ADR guidelines (6.1. group). Both solid-state and solution wastes must be put in leak-proof containers preventing contact with the environment. Additionally., transportation of wastes needs to be made under ADR rules.