Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Carrier
- INCI Name
- Cosmetic Ingredients Functions
- Technologies
- Product Families
Features & Benefits
- Benefit Claims
- Labeling Claims
- Benefits
- Skin whitening
- Assists in the treatment of melasma
- Product Background
Melasma is an acquired chronic hypermelanosis, characterized by brownish macules in the skin's expo- sed areas, especially frontal and malar regions. It affects both sexes, with greater incidence in women, especially pregnant women. It occurs in all races, particularly in individuals with high skin phototypes, who live in areas with high rates of ultraviolet (UV) radiation. UV radiation is an important factor involved in the peroxidation of lipids in the cell membrane, with the release of free radicals, which would stimulate melanocytes. There are many factors involved in the etiology of the disease: genetic influences, exposu- re to UV radiation, pregnancy, hormonal therapies, cosmetics, sun-sensitizing drugs, endocrinopathies, stress, among others.
Tranexamic (trans-4-(Aminomethyl)cyclohexanecarboxylic) acid blocks the conversion of plasminogen (present in epidermal basal cells) into plasmin by inhibiting the plasminogen activator. This activator is responsible for the increased activity of melanocytes in vitro and blocking this effect may be the paracri- ne mechanism for reducing melasma hyperpigmentation. Thus, tranexamic acid has been studied as an effective alternative for the treatment of melasma.
- Use Encapsulated Active Ingredients and Ensure
- Stability Improvement
- Use of sensitive active ingredients (without refrigeration)
- Increased Solubility
- Prolonged release
- Increased effectiveness
- Increased compability in the formulation
- Occlusion of odors
- Increased skin permeation
- Reduced dose
Applications & Uses
- Markets
- Applications
- Use Level
- 2.0 - 10.0
- Usage
- Anionic, nonionic and cationic emulsions
- Anionic and nonionic polymers.
Properties
- Physical Form
- Appearance
- Milky liquid colored Beige to Salmon
- Odor
- Characteristic
- Typical Properties
Value Units Test Method / Conditions pH Stability 3.0 - 7.0 - - pH Value 5.0 - 7.0 - - Relative Density 0.9 - 1.1 g/ml - - Dispersibility
Dispersion of encapsulated active ingredients in water.
- Compatibility
The product has been tested in anionic, nonionic and cationic emulsions and with anionic and nonionic polymers, demonstrating compatibility with the tested bases. For water solutions of low viscosity, the use of a suspending agent or shaking before use is indicated.
- Incompatibility
Enzymes, Sodium chloride, Ethanol and other Organic solvents.
Technical Details & Test Data
- Nanovetores Encapsulation Technology
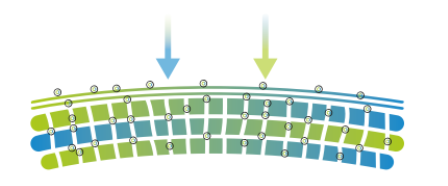
Multifunctional Lipid Particles that promote hydration and extended effect.

Active Ingredient Protection against oxidation resulted from interaction with external environment and other components of the cosmetic formulation.
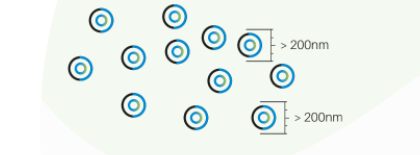
Monodispersity, that ensures control of the particle size, providing adequate permeation to its proposed action.

Secure particles larger than 200nm, biocompatible and biodegradable.

Enzymatic Specific Release Trigger, where enzymes present on the skin disintegrate particles, releasing the active ingredient specifically where it needs to act.
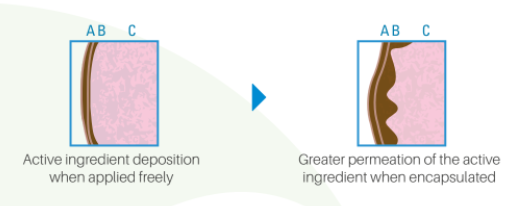
Greater Permeation on the contact surface due to the small size of the capsule.
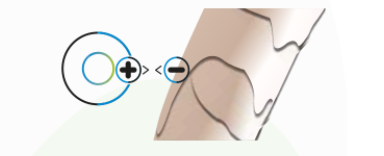
Surface Charge Control of the particle, promoting greater affinity with the contact surface.

Water Base. Active ingredients are manufactured without the use of organic solvents, ensuring safety for users and the environment.
Storage & Handling
- Storage
Keep in temperature between 20-25 °C.