Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Chemical Family
- Fillers Included
- Polymer Name
- Plastics & Elastomers Functions
- Technologies
- Product Families
- Graphene Nanofibers
Carbon Nanofibers is a material belonging to the family of graphene-based materials (GBM). We offer a fully characterized product with well-defined properties and high-quality standards aimed to help researchers of different fields to obtain reproducibility and replicability on their results and boost the outstanding potential of this material.
Features & Benefits
Applications & Uses
- Markets
- Applications
- Plastics & Elastomers End Uses
- Plastics & Elastomers Processing Methods
- Applications
In addition to its regulatory compliance for selected applications, this product emphasizes good impact resistance, surface quality, and increased density. All these characteristics are important in our material to facilitate processability to produce complex geometries, as:
- Medical device housing, respiratory devices: elements or spare parts.
- Digital Anatomy: to create real human anatomy models for surgical preparation and testing of procedures with students.
- For dispensers, pillboxes, and medicine containers. Protective cases.
- Protective packaging and custom packaging systems can provide patient flexibility by incorporating multiple medical devices or medications into one the only package.
The use of a given application must be previously tested by the user, to determine its suitability.
- Recommendations for Use
- Processing temperature range: 235-250 °C. The processing temperature is directly related to the printing speed. The faster the processing speed is, the higher the temperature of the print fuser should be.
- Recommended bed temperature: 90-100 °C. If the adherence to the building platform is weak, the use of products to improve the adhesion between the part and the machine is recommended.
- Layer fan speed: 0%
- Heated chamber temperature: not necessary (if available, use it at more than 30 °C). The use of totally closed printers is recommended to prevent internal stresses produced by the cooling of the parts from causing undesired breakage or deformation.
- Drying conditions: 80 °C for at least 4 hours. Drying of the material is recommended if signs of moisture are detected in the print. Place the filament in the sealed bag with the built-in desiccant after each use.
- Printing speed: 30-60 mm/s. This parameter will depend on the final quality that is expected to be achieved as well as the stability of the printing machine itself.
- Nozzle size: 0.4-0.6 mm. To prevent excessive wear on extrusion components, special hardened or stainless steel nozzles should be used.
- Due to the reinforcement found in the material itself, this can lead to clogs in the nozzles. To eliminate this problem, filament retraction should be used cautiously, even eliminating it.
Properties
- Color
- Physical Form
- Mechanical Properties
Value Units Test Method / Conditions Adhesion Between Layers 3D Max F 242.0 N - Charpy Impact Strength (at 23°C) 16.1 kJ/m² ISO 179-1Eu Elongation (at Break) 10.23 % ISO 527 Elongation (at Yield) 2.0 % ISO 527 Flexural Modulus 2686.65 Mpa ISO 527 Tensile Modulus 1003.14 Mpa ISO 527 Tensile Strength 41.04 MPa ISO 527 - Typical Properties
Value Units Test Method / Conditions Layer Fan Speed 0.0 % - Nozzle Size 0.4 - 0.6 mm - Printing Speed 30 - 60 mm/s - Processing Temperature Range 235 - 250 °C - Recommended Bed Temperature 90 - 100 °C - - Other Properties
Value Units Test Method / Conditions Bed Temperature 90 - 110 °C - Injection Molding-Melt Temperature 235 - 250 °C - Softening Temperature 99.0 °C Vicat Velocity 30 - 60 mm/s - - Material Specifications
Value Units Test Method / Conditions Hardness 81.3 Shore D - Specific Gravity 1.12 g/cc - - Material Properties
The main properties of this product are:
- Surface quality and structural stability.
- High resistance to chemical agents.
- Highly hydrophobic material that inhibits the growth of bacteria, viruses, algae, or fungi.
- Improves the quality of the polymer without affecting its mechanical properties such as tensile strength.
- The nanofiber blocks the contraction, deformation, and lifting of the layer, so it gives a structure and density that facilitates the processability to produce complex geometries.
Regulatory & Compliance
- Certifications & Compliance
- Biocompatibility
- The original polymer meets the requirements of the FDA-modified ISO 10993, Part 1 “Biological Evaluation of Medical Devices” tests with human tissue contact time of 30 days or less and has been tested according to certain tests under ISO 10993-1.
- Tests performed on the polymer: Physicochemical Test for Plastics, Metals Analysis by ICP, Citotoxicity - MEM Elution (ISO 10993-5), Sensitization - Kligman Maximization (ISO 10993-10), Irritation - Intracutaneous Injection (ISO 10993-10), Material-Mediated Rabbit Pyrogen Test (ISO 10993-11), Hemolysis - Rabbit Blood (ISO 10993-4), Genotoxicity - Ames Reverse Mutation (ISO 10993-3) and Intramuscular Implantation (ISO 10993-6).
Technical Details & Test Data
- Antimicrobial Effect
- The antimicrobial effect of graphene-based materials has been widely demonstrated in various investigations. The graphene oxide and the graphene nanofiber are safe and has a high purity, in terms of contaminants such as sulfates or metals (such as iron or manganese), all of which have concentrations below <0,1%.
- The main mechanism of this effect is based to generate reactive oxygen species (ROS) agents such as H2O2, OH-, and O2 capable of destroying the respiratory system of microorganism and specifically attacks the lipid membrane of the coronavirus.
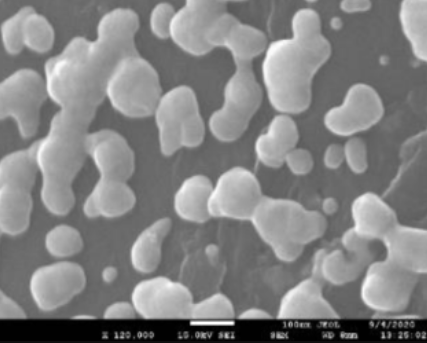
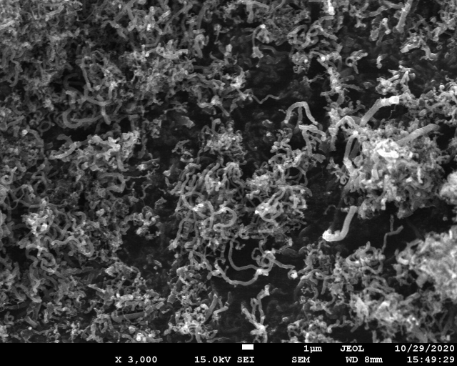
- Test Performed on 3D Printed Specimen
Upper and lower layer 3+3 Intermediate layer 3 Filling 100% Tensile Tester Specimen UNE EN ISO 527 1BA Flexural Test Specimen UNE EN ISO 168 (mm) 80x10x4 - Scanning Electron Microscope (SEM)
Graphene Nanofiber (GNF)
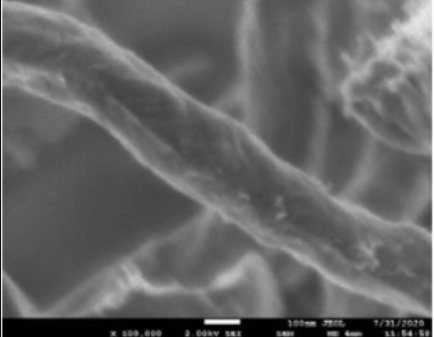
Graphene Oxide (GO)
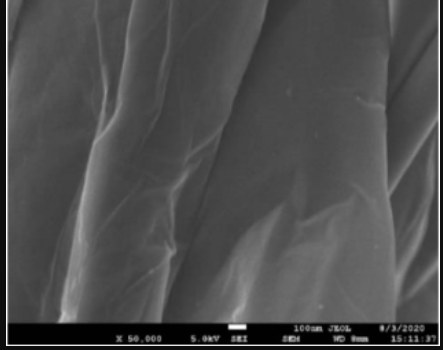
Silver (Ag) + Graphene Oxide