Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Carrier
- Chemical Name
- Country of Origin
- INCI Name
- Ingredient Origin
- Cosmetic Ingredients Functions
- CAS No.
- 477773-67-4
- EC No.
- 686-756-8
- Technologies
- Product Families
Features & Benefits
- Benefit Claims
- Labeling Claims
- Key Features
- SkinBrite-30™ is a water soluble, diamide salt of azelaic acid that improves the technical characteristics for formulation.
- Azelaic acid itself has very poor water solubility, however, it is found in many grain proteins and is a beneficial component of the skin.
- Azelaic acid in the skin is a signaling compound for inflammation and induces the production of salicylic acid.
- This Potassium Azeloyl Diglycinate (PAD) solution is a safe, highly effective and multifunctional cosmetic ingredient.
- As the diamide potassium salt, SkinBrite-30™ is an ingredient that resolves many of the formulation difficulties of azelaic acid.
- SkinBrite-30™ offers water solubility, requires less dosage, has beneficial effects against inflammation, lightens skin and controls sebum without drying out the skin.
Applications & Uses
- Markets
- Applications
- Application Format
- Skin Care Applications
- Treatment Product Applications
- Application pH Range
- 5.5 - 7.5*
- Use Level
- 3.0 - 10.0 %
- Applications
SkinBrite-30 is recommended for controlling sebum, skin lightening and skin moisturization. By helping to bind moisture in the skin, SkinBrite-30 can improve skin elasticity. SkinBrite-30 is used in multifunctional anti-aging formulations, skin whitening creams and lotions, acne prone treatments and facial moisturizers.
Properties
- Physical Form
- Solubility
- Appearance
- Colorless or light yellow transparent liquid
- Soluble in
- Water
- Chemical Composition
Value Units Test Method / Conditions Potassium Azeloyl Diglycinate Content 28 - 32 % - Water Content 67.4 - 71.4 % - Diazolidinyl Urea Content 0.5946 % - lodopropynyl Butylcarbamate Content 0.0054 % - - Specifications
Value Units Test Method / Conditions Activity Content 28.0 - 32.0 % - pH Value (at 20°C) 6.5 - 7.5 - - - Heavy Metals
Value Units Test Method / Conditions Arsenic Content max. 2 ppm - Cadmium Content max. 5 ppm - Lead Content max. 10 ppm - Mercury Content max. 1 ppm - - Note
* - Not suitable for products with low pH
Regulatory & Compliance
- Chemical Inventories
Technical Details & Test Data
- Efficacy Testing Information
In-Vivo Testing of Sebum Normalization Effect:
A sebumeter was used to test sebum levels on the forehead, nose and chin of five volunteers with oily, acne-prone skin who applied a 3% PAD solution (eq. 10% SkinBrite-30™) solution twice daily for 3 weeks.
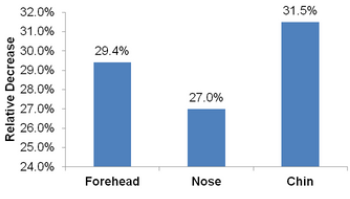
RESULT: PAD significantly reduced sebum in all facial areas tested.
In-Vivo Testing of Moisturization Effect:
Five volunteers with low moisture applied a 3% PAD solution (eq. 10% SkinBrite-30™) to their faces twice daily for 3 weeks. Skin moisturization increased by an average of 12.7% on the forehead and 8.2% on the cheek area.
In-Vivo Testing of Skin Lightening Effect:
The skin of five volunteers with localized hyperpigmentation spots was tested both on and off the spot with a treatment containing 3% PAD (eq. 10% SkinBrite-30™) applied twice daily for 3 weeks. PAD improved hyperpigmented skin color.
- In-Vivo Testing of SkinBrite-30™ to Show Regulation of Sebum Secretion
Five volunteers with oily and acne prone skin used a treatment containing 3% SkinBrite-30 twice daily over three weeks. Sebum content of the forehead, nose and chin was measured initially and after three weeks.
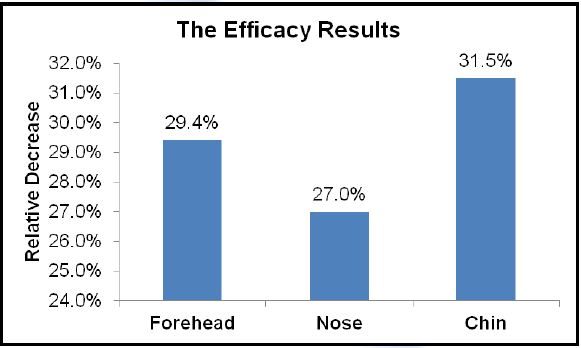
SkinBrite-30 can help regulate sebum secretion in oily and acne prone skin. These efficacy results show that sebum was reduced by 29% on the forehead, 27% on the nose and 32% on the chin.
- In-Vivo Testing of SkinBrite-30™ to Show Skin Lightening
The hands of five volunteers with localized hyperpigmented spots were tested both on and off the localized spot with a treatment containing 3% SkinBrite-30, twice daily over three weeks. Initial skin color was compared to the treated area after three weeks. Again, test sites included the hyperpigmented spots and sites beyond the hyperpigmentation areas. Hands with hyperpigmentation but with no treatment were also monitored.
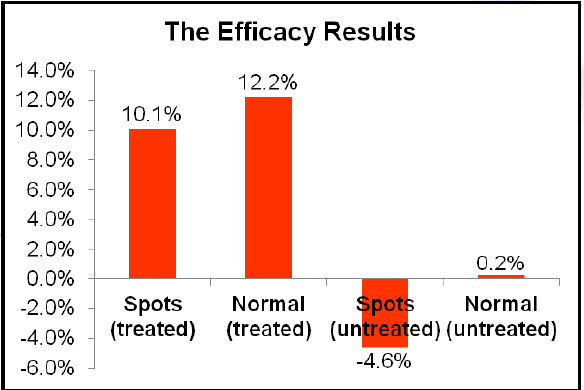
Using SkinBrite-30 at 3% improves hyperpigmented skin color by 10%. On areas without hyperpigmentation, skin color improves by 12%.
- In-Vivo Testing of SkinBrite-30™ to Show Moisturization
A 3% SkinBrite-30 formulation was applied to the forehead and cheek area of five volunteers twice daily over three weeks. Moisturization and skin suppleness was tested initially and after three weeks.
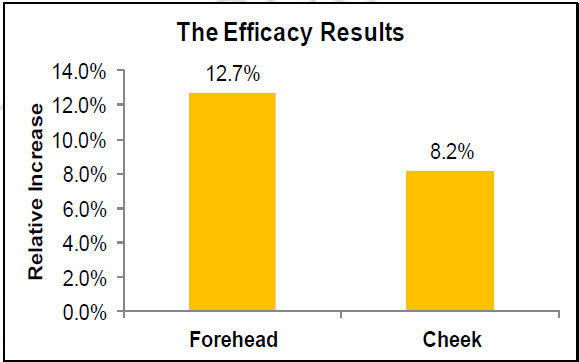
Skin moisturization increased by 13% on the forehead and 8% on the cheek.
Storage & Handling
- Shelf Life
- 3 years
- Storage Conditions
Keep at room temperature away from light