Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Ingredient Name
- Mineral Type
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- CAS No.
- 27214-00-2, 126-95-4, 1336-00-1, 58409-70-4
- EINECS No.
- 248-328-5, 204-813-3, 215-643-4, 261-240-1
- Ingredients
- Calcium Glycerophosphate (CGP)
- Technologies
- Product Families
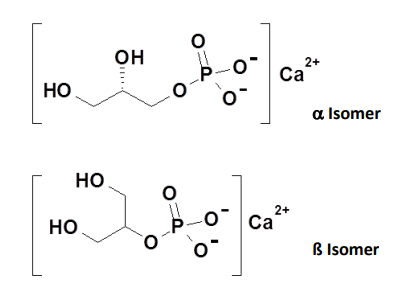
- Chemical Structure
Features & Benefits
- Food Ingredients Features
- Features
A “3 IN 1” SALT
- It supplies calcium, glycerol and phosphorus.
Double Interest
- This type of calcium salt has a physiologic and a metabolic interest due to the glycerophosphate, which is absent in inorganic calcium salts
A Superior Way
- As part of nutraceuticals or health food, GIVOCALTM is a highly bioavailable source of calcium.
Scientific Evidences
- Calcium glycerophosphate shows excellent absorption. Some other clinical studies show positive effects on bone and teeth development.
A Chelate
- Glycerophosphate is an excellent vector for delivering calcium into the GI tract.
- Health Claims For GIVOCAL
- Calcium confributes fo normal blood clofting
- Calcium contributes to normal energy-yielding metabolism
- Calcium contributes to normal muscle function
- Calcium contributes to normal neurotransmission
- Calcium contributes to the normal function of digestive enzymes
- Calcium has a role in the process of cell division and specialization
- Calcium is needed for the maintenance ot normal bones
- Calcium is needed for the maintenance of normal teeth
Applications & Uses
- Markets
- Applications
- Food & Nutrition Applications
- Applications
- As an additive for dietetic purposes (substance for mineral contribution)
- As an acid neutralizer in food and beverages
- Used in chewing gum to give dental health benefits
Properties
- Physical Form
- Solubility
- Molecular Weight
- 210.1
- Taste
- Neutral
- Odor
- Neutral
- Appearance
- Powder, Fine white powder
- Soluble in
- Water, Water
- Insoluble in
- Alcohols (Glycerol, Ethanol)
- Typical Properties
Value Units Test Method / Conditions Density (at 20°C) 0.55 g/cm3 - Theoretical calcium content 19.05 % - Water solubility (20°C) 16.67 g/L - Water solubility (hot water) 1.43 g/L - Calcium Content 19.1 % - Phosphorus Content 14.6 % - Calcium/Phosphorus ratio 1.3 - -
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
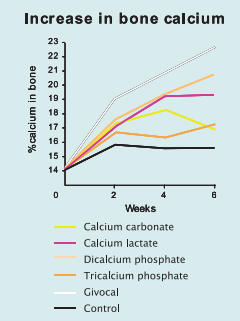
- Bioavailability
Most calcium compounds given orally as a source of calcium are soluble in gastric acid but are converted mostly into insoluble calcium carbonate in the duodenum so that only a fraction of the calcium is available for absorption. Absorption of calcium salts therefore depends greatly on their solubility and stability in a wide pH range. A study on the capacity of acidity neutralization made on several calcium salts shows that GIVOCALTM has the smallest buffer power and then it is the best solubilized in the stomach. It also stays solubilized in the small intestine. These results were confirmed by an in vitro study made on the gastrointestinal model TIM-1 of TNO laboratory (The Netherlands) which compared the absorption of GIVOCALTM and calcium carbonate. With the same amount of calcium ingested (introduced into the system), GIVOCALTM permits a better absorption of calcium: 4 times more calcium is absorbed with GIVOCALTM than with calcium carbonate (internal results). Moreover, a recent study made on rats shows that, with the same amount of calcium ingested, GIVOCALTM has twice as less of calcium excretion than calcium carbonate (internal results). These results suggest that GIVOCALTM has a higher bioavailability than calcium carbonate. Other in vitro or in vivo studies show that GIVOCALTM allows a good assimilation of calcium by the bone tissue, has a cariostatic effect on teeth and shows a beneficial action on the nervous system (internal results).