Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Carrier
- INCI Name
- Ingredient Origin
- Cosmetic Ingredients Functions
- EINECS No.
- 231-791-2, 200-289-5
- Technologies
- Product Families
- Origin
- Glycerin - Vegetable
- Aqua - Mineral
- Hydrolyzed Rhodophycea Extract - Vegetable
Features & Benefits
- Benefit Claims
- Labeling Claims
- Cosmetic Benefits
- Immediate soothing and calming effect for a sensation of comfort
- Reduces and calms erythrosis, giving less redness
- Limits heating and flushes sensations
- Decreases wrinkles and prevents from premature aging
- (data available on demand)
- Rebalances microbial diversity
- Action
- Limits neurogenic inflammation
- Maintains microbiota diversity linked with a better equilibrum
- Enhances genus and species linked with a healthy skin
- Calms erythrosis
- Decreases sensitive skin symptoms
- Product Highlights
Today, 50% of women in Europe, USA and Japan declare to have a sensitive and reactive
skin. Their skin overreacts to environmental factors with sensations of tingling, heat,
pricking, burning, pain, itch with or without erythema. These factors can be external
(pollution, UV, chemical agents), or internal (stress, hormones, emotions), but in all cases
the skin is discomfortable, and visibly marked. It appears that skin microbiota is also
linked with sensitive skin.
Faced with this huge challenge, GREENTECH developed an active capable of protecting
the skin against deleterious effects of exposome : ExpoZen®.
Applications & Uses
- Skin Care Applications
- Cosmetic Use
- Sensitive and reactive skin products
- Urban skin care
- Daily skin care
- Anti-aging range
- Baby care
- Skin microbiota regulator
- Formulations
- Concentration for use: 3%
- Caution for use: Add in formulations at 35-40°C, while cooling or at any time in cold preparation.
- pH for use: 4.0 - 8.0
Properties
- Physical Form
- Odor
- Characteristic
- Appearance
- Limpid to opalescent liquid
- Soluble in
- Water, Glycerin
- Partially Soluble in
- 10% in water
- Typical Properties
Value Units Test Method / Conditions Natural Content 0 % - Natural Origin Content 100 % - - Physico-Chemical Properties
Value Units Test Method / Conditions Sulfation Degree 20 - 40 % GT099 Total Sugars (exp. eq. galactose-sulfate) min. 6.5 % m/V GT044 pH Value (Direct) 2.9 - 4.8 - GT005 - Microbiological Values
Value Units Test Method / Conditions Detection of Enterobacteria Absence - ISO 18415 Enumeration of Mesophilic Aerobic Bacteria max. 100 ufc/g - cfu/g ISO 21149 Enumeration of Yeasts and Molds max. 100 ufc/g - cfu/g ISO 16212
Regulatory & Compliance
- Certifications & Compliance
- REACH Status
INCI Concentration REACH status Glycerin 72 % Glycerin exempted: annexe V point 9 Aqua 14.5 – 21.5 % Water exempted annex IV Hydrolyzed Rhodophyceae
Extract6.5 – 13.5 % Exempt: < 1 ton/year (dry matter)
Technical Details & Test Data
- Impact of Exposome on Skin
HOW DOES EXPOSOME IMPACT SKIN?
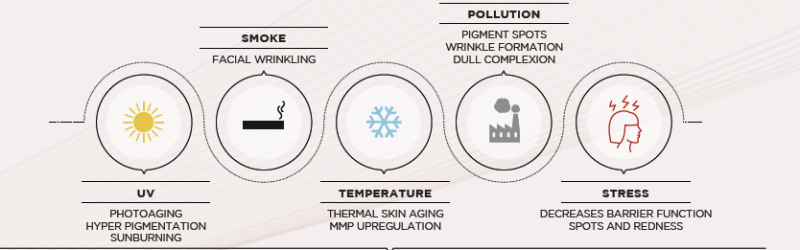 PREMATURE SKIN AGING
PREMATURE SKIN AGING
SENSITIVE SKINBiological Mechanisms involved in sensitive skin

Abbreviations
- TRPV-1: Transient receptor potential vanilloid type 1
- NK1-R: Neurokinin-1
- VEGF: Vascular endothelial growth factor
Link between sensitive skin and microbiota?

- ACNE: Association of some strains of cutibacterium acnes with acne
- ROSACEA: Increased corynebacteria on the skin
- DANDRUFF: Unbalance between staphylococcus and cutibacterium acnes species
- PSORIASIS: Firmicutes most abundant phylum whereas actinobacteria underrepresented
- Proven Efficacy - In Vitro Test
Expozen® Limits Inflammation And Neurogenic Inflammation
 On human explants. Treatment with ExpoZen® at 5% during 6 days.
On human explants. Treatment with ExpoZen® at 5% during 6 days.
** p<0.01 vs controlTRPV-1 facilitates neurogenic inflammation and mediates the induction of pro inflammatory cytokines and MMPs by UV irradiation in keratinocytes.

On human explants. Treatment with ExpoZen® during 6 days.
Immunodetection and quantification of stained epidermis area.
*** p<0.001 vs controlExpoZen® reduces the activation of TRPV-1.
Exposome thins the epidermis; the skin becomes less resistant to shearing forces and more vulnerable to agressions.
 On reconstructed epidermis.
On reconstructed epidermis.
Treatment with ExpoZen® at several concentrations during 24 hours.
* p<0.05 vs controlExpoZen® induces a significant thicker epidermis.
- Proven Efficacy - Clinical Study
Expozen® Maintains The Diversity Of Skin Microbiota And Reduces Specific Species
Characterization of Reactive and Sensitive Skin Microbiota (At D-1)

WITH ExpoZen® (at D28)

Reduces strains involved in inflammation and redness
(C. kroppenstedtii) and increases beneficial strains (S. epidermidis)ExpoZen® HAS VISIBLE EFFECTS
MEASURED BY VIDEOCAPILLAROSCOPE

* p<0.05 vs D0

ExpoZen® reduces erythrosis
ExpoZen® IMPROVES THE SKIN COMFORTThe stinging test is a tool in the assessment of sensitive skin and the soothing potential of cosmetics.
90% OF WOMEN FELT A SOOTHING EFFECT 5 MINUTES AFTER APPLICATIONThe Sensitive Scale (SS-10)# enables to measure the severity of skin sensitivity and thus permits the measurement of variations pre- and post-treatment.

Double blind test, 25 volunteers, from 22 to 56 years old. ExpoZen® 3% versus placebo. Twice daily application during 28 days on a sensitive and reactive skin (Stinging test score > 3).
* p<0.05, *** p<0.001 vs D0.Words cloud chart reported by the women at D28 regarding their comments concerning ExpoZen®.

ExpoZen® reduces the reactive and sensitive skin symptoms.
Safety & Health
- Toxicological Tests
Tests Method Tested Concentration Results Skin Irritation Patch Test 3 % Slightly
irritantEye Irritation Het cam CFIO Test 3 % Slightly
irritantSensitization Marzulli-Maibach 5 % Not sensitizing Phototoxicity 3T3 cells 5 % Not phototoxic Mutagenicity Test Ames test 5 % Not mutagenic
and not promutagenic
Storage & Handling
- Shelf Life
- 24 months
- Storage
15 / +25°C
Keep in the original packaging, without opening, in the recommended storage conditions.
Advice before use : Agitation