Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Active Component
- Ingredient Name
- Ingredients
- Olive Oil, Arabica Gum, CBD Powder
- Technologies
- Product Families
- Composition
20% active cannabinoid load; all natural, clean-label carriers and non- synthetic emulsifiers
- Derived from
Pure isolates or refined hemp distillates derived from whole hemp plants
Features & Benefits
- Labeling Claims
- Food Ingredients Features
Applications & Uses
- Markets
- Applications
- Food & Nutrition Applications
- Applications
- Water-soluble powder is ideal for ready-to-drink mixes, stick packs, bulk powder tubs, shots, foods, beverages and supplements.
- Each application is very different : The stability and performance of the cannabinoid ingredients are highly dependent on the other ingredients in the product. Factors like pH, sugar content, manufacturing conditions and container type and size can all play a major role in flavor and stability.
Properties
- Physical Form
- Solubility
- Appearance
- Fine, free-flowing, white powder
- Flavor
- Neutral to slightly bitter
- Odor
- Neutral to faint terpenoid
- Soluble in
- Water
- Typical Properties
Value Units Test Method / Conditions Moisture Content* 2.0 % Karl Fischer method Consistency Fine powder - - - Microbiological Values
Value Units Test Method / Conditions Aerobic Plate Count* max. 100 cfu/g - Escherichia Coli* Absent per 10g - Enterobacterial Count* max. 10 MPN/g - Yeast Count* max. 100 cfu/g - Mold Count* max. 100 cfu/g - - Heavy Metals
Value Units Test Method / Conditions Arsenic Content max. 10.2 ppb Dry Basis Cadmium Content max. 5.1 ppb Dry Basis Lead Content 13.5 ppb Dry Basis Mercury Content max. 5.1 ppb Dry Basis - Cannabinoid Content
Value Units Test Method / Conditions CBDVA max. 0.00255 % Dry Basis CBDV 0.0424 % Dry Basis CBDa max. 0.00255 % Dry Basis CBGa max. 0.00255 % Dry Basis CBG max. 0.00255 % Dry Basis CBD 21.7 % Dry Basis THCV max. 0.00255 % Dry Basis CBN max. 0.00255 % Dry Basis Δ9-THC max. 0.00255 % Dry Basis Δ8-THC max. 0.00510 % Dry Basis THCA max. 0.00255 % Dry Basis CBC max. 0.00255 % Dry Basis THCVA max. 0.00255 % Dry Basis CBNA max. 0.00255 % Dry Basis CBCA max. 0.00255 % Dry Basis CBL max. 0.00255 % Dry Basis Total Cannabinoids 21.8 % Dry Basis Total THC (THC + (THCA x 0.877)) max. 0.00255 % Dry Basis Total CBD (CBD + (CBDA x 0.877)) 21.7 % Dry Basis - Note
* This analysis or component is not ISO accredited.
Regulatory & Compliance
- Compliance
- United Kingdom: Novel Foods compliance in process
- European Union: Novel Foods compliance in process
- Produced in cGMP facilities
- Validated using trusted third-party accredited laboratories
Technical Details & Test Data
- Water-soluble Powder Has Demonstrably Better Bioavailability
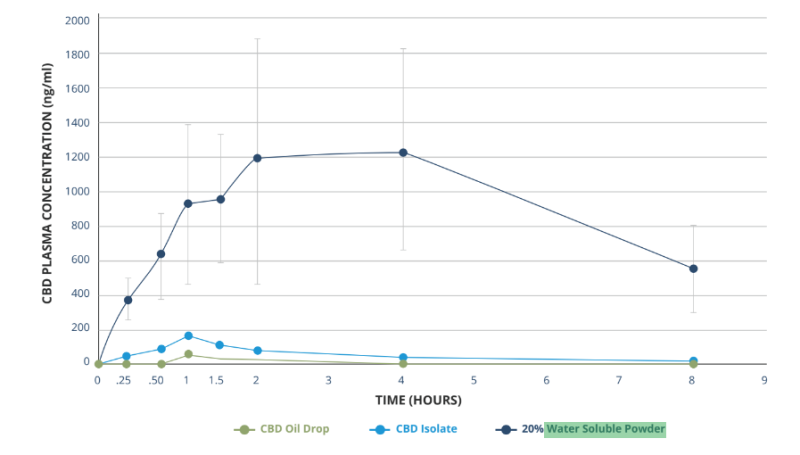
Plasma pharmacokinetic (PK) study in rats following single oral administration of three (3) cannabidiol formulae
As shown in the graph, this product format is more bioavailable than cannabinoids are on their own, which allows for broad use in liquid and powder formulations - from shots and beverages to food, supplements and pet applications.
Storage & Handling
- Shelf Life
- 24 months