Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Ingredient Name
- Ingredient Origin
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Features & Benefits
- Benefit Claims (Health)
- Labeling Claims
- Food Ingredients Features
Applications & Uses
- Markets
- Applications
- Food & Nutrition Applications
- Use Level
- 50-100 mg/day
Properties
- Physical Form
- Solubility
- Appearance
- Light yellow fine powder
- Typical Properties
Value Units Test Method / Conditions Moisture max. 8 % - Water Activity max. 0.92 - - - Microbiological Values
Value Units Test Method / Conditions Total Cell Count min. 1.0 x 10¹² Cells/g - Aerobic Plate Count max. 5000 cfu/g - Coliform Bacteria Absent - - Yeast and Mold max. 300 cfu/g - - Heavy Metals
Value Units Test Method / Conditions Heavy Metals (as Pb)* max. 20 ppm - Arsenic Content max. 2 ppm - - Note
Note : *tested at least once a year.
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- Test Data
Figure. 1 The comparison between probiotics, postbiotics, and ProbioAid® postbiotics.
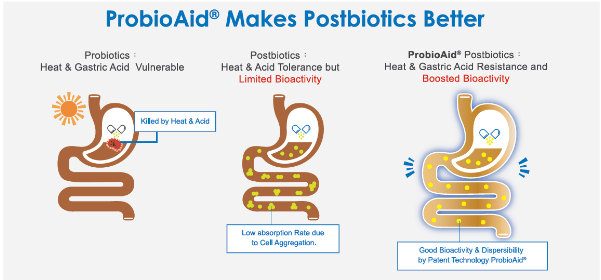
Figure. 2 ProbloAid® postbiotics has higher bioactivity than general postbiotics proven by increasing immune response in serum and spleen.
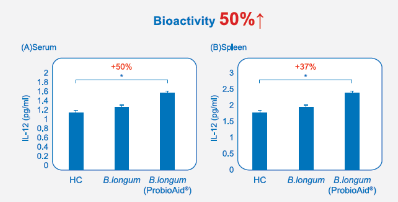
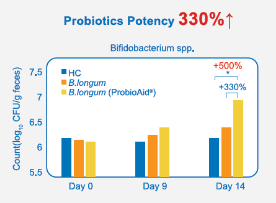
Figure. 3 After consuming ProbioAid® postbiotics, 500% probiotics potency more than control, and 330% more than general postbiotics.
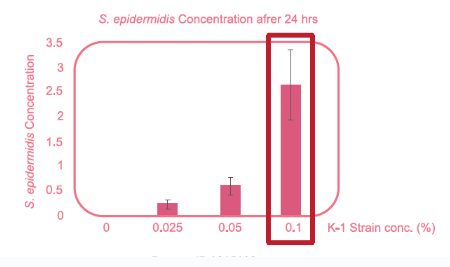
- Technical Function (Optimize Skin Microbiome)
Significant increase of S. epidermis concentration in 24 hrs
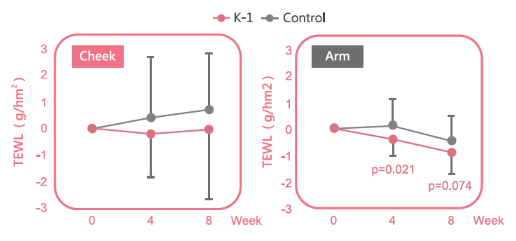
Reduce trans-epidermal water loss level and improve skin barrier function
Packaging & Availability
- Packaging Type
- Packaging
1Kg /Aluminum pack.
Storage & Handling
- Shelf Life
- 36 Months
- Storage and Shelf Life Conditions
Shelf Life
- 36 Months (Stored closed in original sealed package)
Storage Condition
- Be Kept in an airtight container and protection from sunlight, oxygen and moisture.