Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Chemical Name
- INCI Name
- Ingredient Name
- Ingredient Origin
- Vitamin Type
- Cosmetic Ingredients Functions
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
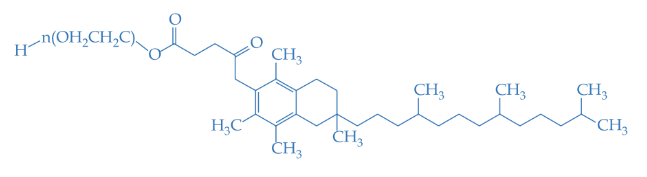
- Molecular formula
- (C₂H₂O)ₙ-C₃₃H₅₄O₅
- Technologies
- Product Families
- Molecular Structure
Features & Benefits
- Benefit Claims
- Benefit Claims (Health)
- Labeling Claims
- Key Features
- Produced with stringent industry requirements, maintaining GMP conditions
- Technology driven process to maintain the supply chain
- Synthesized from natural source of Vitamin E
- No chlorinated solvents used
- Complies as per USP
- Offers high activity and reliable composition
Applications & Uses
- Markets
- Applications
- Dosage Form
- Food & Nutrition Applications
- Treatment Product Applications
- Application
- Emulsifier - TPGS has amphiphilic properties and is therefore used as emulsifier for nutraceuticals and pharmaceuticals (Lehr 2005). Emulsifier for injectable formulations.
- Drug Delivery System -
- Drug solubilizer It directly increases the bioavailability and delivery of poorly soluble drugs
- Protects drugs from crystallization
- Provides Vitamin E or poorly soluble nutraceuticals in liquid dosage form
- Reduces drug sensitivity on skin and tissues
- Acts as a vehicle in semi-dosage form and also for pulmonary (inhalation) dosage form
- Carrier for wound care and treatment
- A thermal binder in melt granulations/ extrusion process
- Absorption enhancer
- Antioxidant
- Vehicle for lipid - based drug delivery - Vitamin E TPGS is an important extension to a range of drug delivery products
- Source of natural Vitamin E - used in pharmaceutical formulations as well as dietary supplements
- Extensively used in solid, topical and ophthalmic dosage forms
Properties
- Physical Form
- Solubility
- Typical Properties
Value Units Test Method / Conditions Acidity max. 0.27 ml - Assay min. 25 % - Heat Capacity 1.7 J/g.K - Heat of Melting 99.8 J/g - Heavy Metals max. 10 ppm - Melting Point 37 - 41 °C - Molecular Weight approx. 1513 - - Solubility in water 20.0 g/100ml - Specific Gravity (at 50°C) 1.06 - - Specific Gravity (at 90°C) 1.03 - - Specific Optical Rotation min. +24 ° - Viscosity (at 50°C) 300 - 400 cp - - Amphiphilic Property
Vitamin E TPGS is amphiphile, that is it has a dual nature. Part of the molecule exhibits hydrophilicity and the other lipophilicity. General accepted view is that the polyethylene glycol portion serves as the hydrophilic polar head (water soluble), while the tocopherol succinate portion serves as the lipophilic alkyl tail (water insoluble).
Regulatory & Compliance
Safety & Health
- Toxicology Information
From toxicology studies, an overall no observed adverse effect level (NOAEL) of 1000 mg/ kg body weight per day can be derived. Vitamin E TPGSs is not genotoxic. Also approved by US- FDA (United States - Food and Drug Administration).
Storage & Handling
- Shelf Life
- 4 Years
- Stability
- Vitamin E TPGS is stable when exposed to oxygen, heat, light, or oxidizing agents. It is unstable to alkali.
- Vitamin E TPGS is known to be stable excipient with a shelf-life of 4 years when stored in the original unopened container at room temperature.
- Vitamin E TPGS is stable under the conditions of heat sterilization.