Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Chemical Family
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Features & Benefits
- Benefit Claims
- Product Information
- With CompactCel® SIL it is possible to produce direct compressed tablets containing excipients and a high content of active ingredients (which are normally incompressible) in one step. A wet granulation step to isolate the active ingredients with an excipient is in most cases not needed. Further-more liquid ingredients can be converted into powders.
- The use of Silica within the compound improves free flowing properties, gives better compaction and a superior adsorptive capacity for increased stability (by adsorbing surrounding liquids or moisture) and an improved dissolution profile.
- CompactCel® SIL increases the hardness and improves the friability of the tablet at lower compression forces. BIOGRUND recommends using 2–5% within the core formulation to achieve best results. The product can be used for pharmaceutical and nutritional applications.
Applications & Uses
- Markets
- Applications
- Dosage Form
- Manufacturing Technology
- Food & Nutrition Applications
- Use Level
- 2 - 5%
Properties
Regulatory & Compliance
- Certifications & Compliance
- Grade
- Regulatory and Quality Aspects
All CompactCel® SIL formulations aredeveloped to meet the official regulatory requirements of the user’s country for pharmaceutical products and for nutritional or dietary supplements.
Technical Details & Test Data
- Friability and Hardness Comparison
The use of Silica within the compound improves free flowing properties, gives better compaction and a superior adsorptive capacity for increased stability (by adsorbing surrounding liquids or moisture) and an improved dissolution profile.
CompactCel® SIL increases the hardness and improves the friability of the tablet at lower compression forces.
Tablet Friability Comparison (n=10)
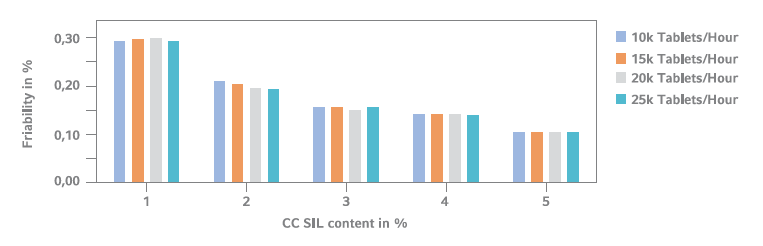
Direct compressed formulation: 50% Glucosamine-Sulfate 2KCl, MCC (42.5–47.5%), 2% NaCMC-CL, CompactCel® SIL (HPC/NaCMC/Silica, 0.0–5.0%) and 0.5% Mg-St. Using already 1% of the dry binding agent in the tablet mass causes friability values lower than 0.3%.
Tablet Hardness Comparison (n=10)
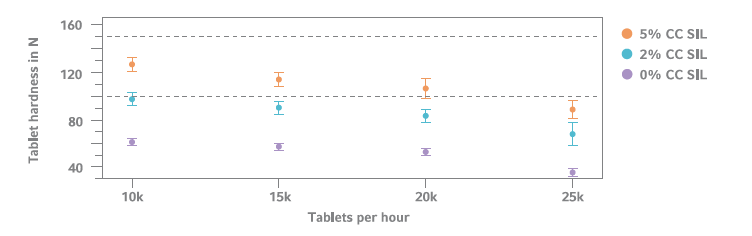
Direct compressed formulation: 50% Glucosamine-Sulfate 2KCl, MCC (42.5– 47.5%), 2% NaCMC-CL, CompactCel® SIL (HPC/NaCMC/Silica, 0.0–5.0%) and 0.5% Mg-St. Optimal values (between 100–150 N) are shown within the dashed area.

