Enhanced TDS
Knowde-enriched technical product data sheet
Identification & Functionality
- Chemical Family
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Features & Benefits
- Product Information
- Regardless of whether a pharmaceutical or nutraceutical grade is required, AquaPolish® E can be formulated with the appropriate polymers (acrylic acid copolymer, HPMC AS or sodium alginate), coloring additives and additional excipients. It is a homogeneous, dry-milled, powdered compound, developed for the coating of all kinds of solid dosage forms.
- Systems formulated with a partially neutralized film-forming polymer make the coating process cost effective because of the high amount of polymer in the ready-to-use compound. It is easily soluble in water, and a reproducible highquality film coating is given. Clear, white and colored preparations can be tailor-made according to customer requirements.
- For nutraceutical applications where vegan formulations are required, the use of the film-forming polymer sodium alginate is suitable.
- AquaPolish® E can be applied by using conventional or modern coating equipment.
Applications & Uses
- Markets
- Applications
- Dosage Form
- Manufacturing Technology
- Food & Nutrition Applications
Properties
Regulatory & Compliance
- Certifications & Compliance
- Grade
- Regulatory and Quality Aspects
AquaPolish E formulations are developed to meet the official regulatory requirements of the user’s country for pharmaceutical products and nutritional or dietary supplements.
Technical Details & Test Data
- Release of Acetylsalicylic Acid from Coated Pellets
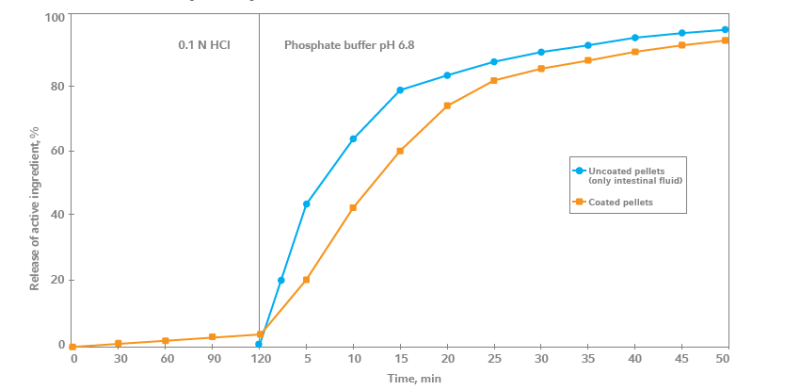
The release of the active ingredient was tested using the coated pellets by placing them
in 0.1 N HCl for a period of 2 hours and afterwards in phosphate buffer (pH 6.8). Pellets
coated with AquaPolish® E based on methacrylic acid-ethyl acrylate copolymer type B.- Product Performance