Knowde Enhanced TDS
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
- Chemical Structure
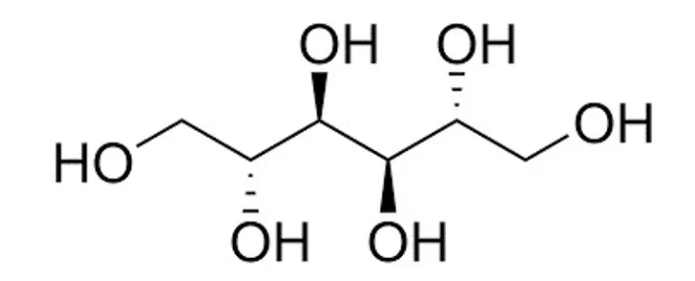
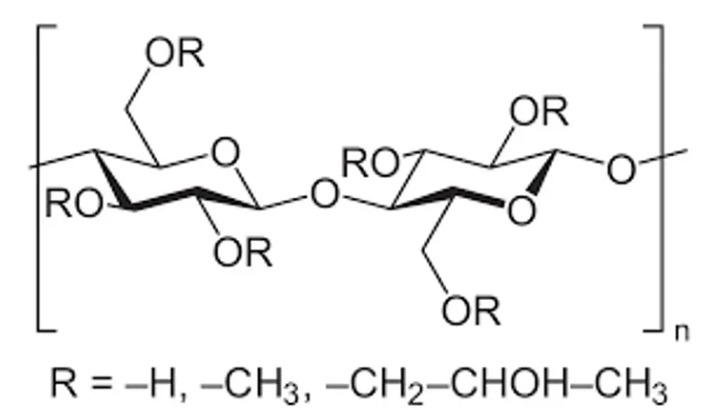
Mannitol Hydroxypropyl methylcellulose
- Morphology

Features & Benefits
- Key Attributes
- Uses multicompendial ingredients
Applications & Uses
- Applications
- Manufacturing Technology
- Product Applications
PEARLITOL® CR-H co-processed mannitol HPMC is a direct compression excipient providing functional properties of binder as well as controlled release to your tablet. PEARLITOL® CR-H can be used in both pharmaceutical and nutraceutical oral dosage forms.
Properties
- Physical Form
- Appearance
- White to yellowish-white powder or granules
- Odor
- Neutral taste, slightly sweet
- Partially Soluble in
- Cold water
- Typical Properties
- Microbiological Values
- Particle Size Distribution
| Value | Units | Test Method / Conditions | |
| Loss On Drying | max. 4.0 | % | - |
| pH | 5 - 8 | - | - |
| D-Mannitol (as is) | 25 - 35 | % | - |
| Hypromellose (as is) | 65 - 75 | % | - |
| Sulfated Ash | max. 1.0 | % | - |
| Bulk Density | 300 - 500 | g/l | - |
| Molecular Weight | 182.2 | g/mol | - |
| Water Content | max. 4 | % | - |
| Average Mean Particle Diameter | 150.0 | µm | - |
| Particle Size Distribution (dv10) | 60.0 | µm | Laser Diffraction |
| Particle Size Distribution (dv50) | 140.0 | µm | Laser Diffraction |
| Particle Size Distribution (dv90) | 290.0 | - | Laser Diffraction |
| Melting Temperature | 165 - 170 | °C | - |
| Powder Flowability | 12.0 | seconds | - |
| Tapped Density | 0.43 | g/cm³ | - |
| True Density | 1.456 | g/cm³ | - |
| Specific Surface Area | 0.8 | m²/g | - |
| Value | Units | Test Method / Conditions | |
| Total Aerobic Microbial Count | max. 1000 | CFU/g | - |
| Total Yeasts and Molds Count | max. 100 | CFU/g | - |
| Escherichia Coli | Not detected | in 1g | - |
| Salmonella | Not detected | in 10g | - |
| Value | Units | Test Method / Conditions | |
| Particle (more than 500microns) | max. 5 | % | Laser |
| Particle (more than 315 microns) | max. 25 | % | Laser |
| Particle (more than 40 microns) | min. 90 | % | Laser |
Regulatory & Compliance
- Certifications & Compliance
- Grade
- Conformity
Meets the requirements of the current monograph of
- European Pharmacopeia MANNITOL (0559) and HPMC
- USP-NF Pharmacopeia MANNITOL and HPMC
- Japanese Pharmacopeia D-MANNITOL and HPMC
- Chinese Pharmacopeia MANNITOL and HPMC
Please contact us for any statement regarding compliance to the General Chapters (elemental impurities, residual solvents, organic volatile impurities, metal catalyst, metal reagent).
Technical Details & Test Data
- Product Test Data
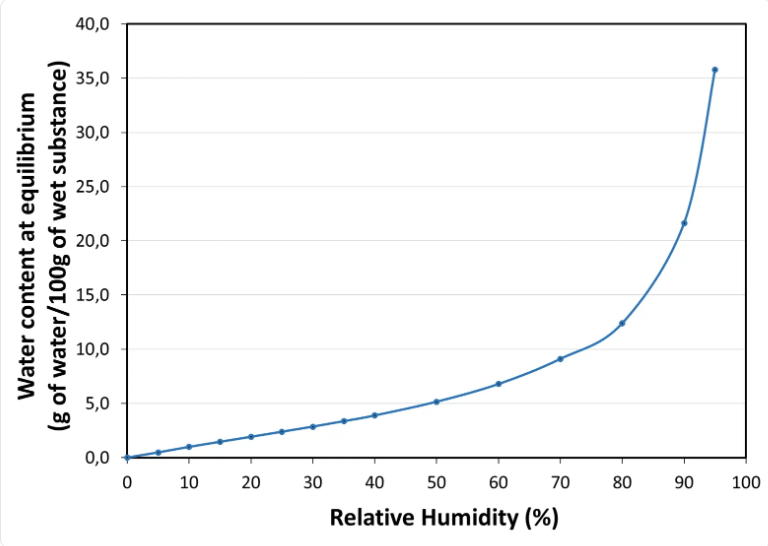
Water sorption isotherm at 20°C
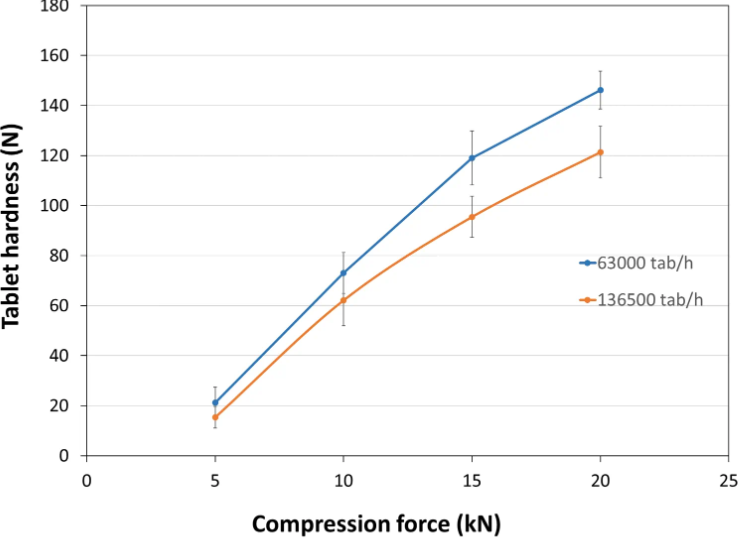
Tablet Hardness
API Release Kinetics
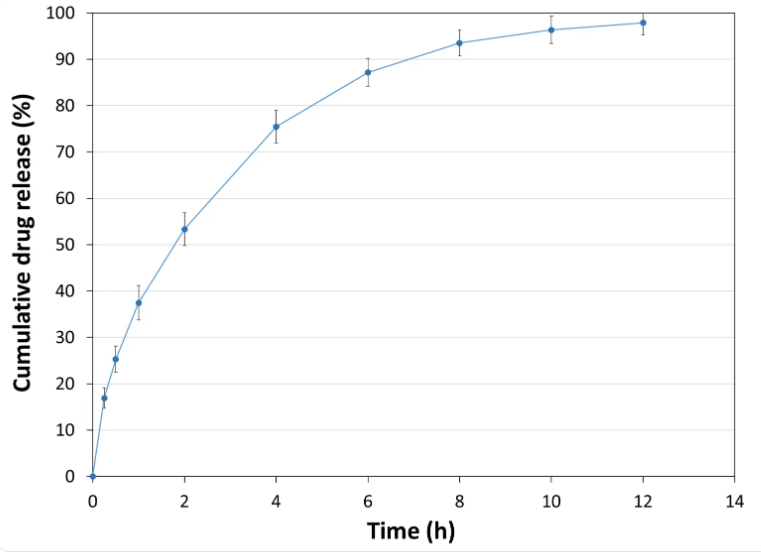
- Experimental Conditions for Compression Behavior
Tablet Press: STYLONE EVO
Production Speed:
30 and 65 RPM (linear punch velocity: 49 and 91 mm/s; simulated KORSCH XL-400 rotary press speed: 63,000 and 136,500 tablets/hour)
Tooling: Diameter 13 mm flat
Formula: PEARLITOL® CR-H / external lubrication with magnesium stearate
Tablet Mass: 400 mg- Experimental Conditions for Sustained Release Behavior
Tablet press: STYL'ONE EVO
Production speed: 30 RPM (linear punch velocity: 49 mm/s; simulated KORSCH XL-400 rotary press speed: 63,000 tablets/hour)
Tooling: Diameter 13 mm flat
Formula: 50% Metformin / 49% PEARLITOL® CR-H / 1% magnesium stearate
Tablet mass: 1,000 mg
Tablet hardness: 109 N
Tablet thickness: 6.8 mm
Dissolution bath: USP II with helical sinker
Release media: pH transition method adapted from US <711> Dissolution Method A
Storage & Handling
- Shelf Life
- 3 years
- Storage Condition
- Manufacturing date: 3 years, in its unopened packaging.
- These dates are indicative and may vary according to packaging type and manufacturing plant. Proper information is shown on labeling and CoA.
- We recommend to preserve the product in its unopened original packaging, preferably protected from wide variations of temperature and humidity.
- Upon opening, use the product as quickly as possible to prevent moisture regain.



